Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
At Boyle's Temperature ; gas behaves ideally for a range of pressure.
New answer posted
3 months agoContributor-Level 10
Two same group on any C-atom of double bond ⇒ (compound does not show geometrical isomerism)
New answer posted
3 months agoContributor-Level 10
Gutta percha is a synthetic rubber and its monomer is isoprene .
Since isoprene has EDG, so it is prepared by cationic addition polymerization
New answer posted
3 months agoContributor-Level 10
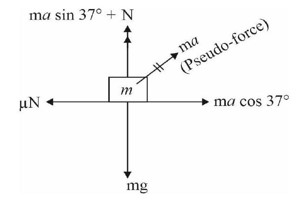
From Reaction
Δn? = 2 – 1 = 1
Kp = Kc (RT)^Δn?
Kp = Kc (RT)¹
Kc = Kp (RT)? ¹
New answer posted
3 months agoContributor-Level 10
Thermal stability of compound is directly proportional to large anion of s-block elements:
T.S ∝ 1/ (PP of cation)
And p.p. ∝ charge ∝ 1/size, So, [T.S. ∝ size ∝ 1/ (charge)]
So, Sr [NO? ]? is highly stable
But Mg (NO? )? ⇒ poor
New answer posted
3 months agoContributor-Level 10
All have same molarity, pH ∝ 1/ (Acidic strength) ∝ Basic strength
H? SO? → Acidic
NH? Cl (Salt of weak base and strong acid)
Acidic but less than H? SO?
NaCl → (Neutral solution)
NaOH → Basic (Strong Base)
So NaOH > NaCl > NH? Cl > H? SO?
New answer posted
3 months agoContributor-Level 10
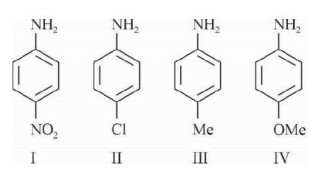
and basic strength ∝ 1/pK? ∝ K? ∝ +R ∝ 1/ (-R) ∝ +I ∝ 1/ (-I)
So basic strength order ⇒ IV > III > II > I, so pK? order is IV < III < II < I
New answer posted
3 months agoContributor-Level 10
? Solvent is H? O, which is in excess
So using m ( molality ) = (x? *1000)/ (x? * (M? )? )
? x? = 0.74 (Mol? = 18 g)
x? = 1 – 0.74 = 0.26 ∴ m = (0.26 * 1000)/ (0.74 * 18) = 19.5
New answer posted
3 months agoIf Hund's rule is violated, then which among the following will become diamagnetic from paramagnetic
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers