Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Number of mol of A reacted = 1*10? = 10?
Number of molecule of A = 10? * 6 *10²³
Quantum efficiency = (6 *10¹? ) / (1.2 *10¹? ) = 0.50
New answer posted
3 months agoContributor-Level 10
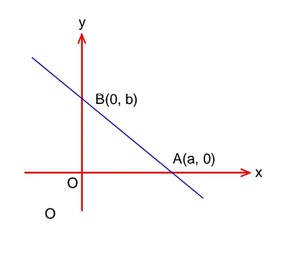
According to question,
Equation of required line is
Obviously B (2, 2) satisfying condition (i)
New answer posted
3 months agoContributor-Level 10
ΔG° = – 2.303RT log Kp = – 2.303* 2 * 300log10? ³
= – 2.303* 2 * 300 * (– 3) = 4145.4cal mol? ¹
New answer posted
3 months agoContributor-Level 10
P? = 1atm
P? = 1atm * 40/100 = 0.40atm
V? = 100 cm³
V? =?
At constant temperature, P? V? = P? V?
So V? = (P? V? ) / P? = (1atm * 100 cm³) / 0.40 atm = 250cm³
Hence, the volume of bulb B = (250 –100) cm³ = 150 cm³
New answer posted
3 months agoContributor-Level 10
As 500g of tooth-paste has = 0.2g of F?
So, 10 g of toothpaste has = (0.2g * 10? g) / 500g F = 400g of F
Hence, concentration in ppm is 400.
New answer posted
3 months agoContributor-Level 10
As 3f-orbital does not exist as value of l cannot be equal to n, hence no electron can be accommodated.
New answer posted
3 months agoContributor-Level 10
ΔH_rxn = ΔH_f (N? O, g) + 3ΔH_f (CO? , g) – (2ΔH_f NO? , g) – 3ΔH_f (CO, g)
= 81 + 3* (– 393) – 2 * 34 – 3 (–110)
= 81 – 1179 – 68 + 330 = – 836 kJ
New answer posted
3 months agoNew answer posted
3 months agoContributor-Level 10
One mole of water is converted to vapour at its boiling point which is 100°C and at 1 atm. For this process ΔG = 0. As phase transformation of water is an equilibrium process and at equilibrium, free energy change is always zero.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers