Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
The displacement S is calculated by integrating the velocity function:
S = x - x? = ∫? ¹ v dt = ∫? ¹ (v? + gt + Ft²) dt = v? + g/2 + F/3
New answer posted
4 months agoContributor-Level 10
The acceleration 'a' is calculated as:
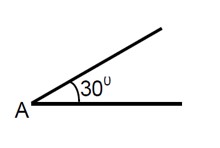
a = (g sinθ) / (1 + I/mr²) = (10 * sin30°) / (1 + 2/5) = 25/7 m/s²
The time 't' is calculated as:
t = 2v / a = (2 * 1) / (25/7) = 0.57 s
New answer posted
4 months agoContributor-Level 10
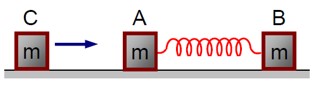
For an elastic collision where C comes to rest, and the compression in the spring is maximum, the velocities of A and B are equal (v). Using the conservation of mechanical energy:
(1/2)mv? ² = 2 * (1/2)mv² + (1/2)kx²
This gives the maximum compression x as:
x = v? * √* (m / 2k)*
New answer posted
4 months agoContributor-Level 10
In an aqueous medium, all alkali metal cations are converted into their hydrated form. More intensely positive cations have more hydration, and hence the size of the cation increases after hydration. Therefore, the Li? cation has a larger size in hydrated form and the least conductivity. The order of conductivity of alkali metal ions is: Li? < Na? < K? < Rb? < Cs?
New answer posted
4 months agoContributor-Level 9
For constant acceleration, a,
x = ut + (1/2)at² ⇒ x-t graph is parabola.
v = u + at ⇒ v-t graph is straight line with positive slope.
New answer posted
4 months agoContributor-Level 10
Except for option (D), all are the important characteristics of D? O. D? O is useful for the isotopic leveling effect in reaction mechanisms. The dielectric constants of H? O and D? O are respectively 80 and 60.
New answer posted
4 months agoContributor-Level 10
Due to the larger size of the Cl-atom, the addition of an electron in the outermost orbit produces less electronic repulsion with respect to the F-atom. The following are the electron gain enthalpies in (KJ) of halogens:
F → -328, Cl → -349, Br → -325, I → -295
New answer posted
4 months agoContributor-Level 9
Layers of atmosphere are in order of (from bottom) troposphere, stratosphere, mesosphere, thermosphere.
New answer posted
4 months agoContributor-Level 9
For adiabatic process CD,
W = (P? V? - P? V? ) / (γ - 1) = (100 * 4 - 200 * 3) / (1.4 - 1) = -500J
New answer posted
4 months agoContributor-Level 10
A central atom having two lone pairs and three bond pairs reflects sp³d hybridization and a corresponding T-shaped geometry.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers