Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
Given the decay equation A = A? e^ (-bt/m):
-bt/m = ln (A/A? )
Solving for b:
b = (-m/t? ) * ln (A/A? ) = (-1 / (2 * 60) * ln (6/12) = 5.775 * 10? ³ kg/s
New answer posted
4 months agoContributor-Level 10
n (s) = 6² = 36
E = { (1, 1), (1, 2), (1, 3), (1, 5), (1, 7), (2, 1), (2, 2), (2, 3), (2, 5), (3, 1), (3, 2), (3, 3), (3, 5), (5, 1), (5, 2), (5, 3), (7, 1)}
∴ n (E) = 17
Required prob. = n (E) / n (S) = 17 / 36
New answer posted
4 months agoContributor-Level 10
The bulk modulus B is calculated as:
B = -ΔP / (ΔV/V) = (ρgh) / (ΔV/V) = (10³ * 9.8 * 2 * 10³) / (1.36 * 10? ²) = 1.44 * 10? N/m²
New answer posted
4 months agoContributor-Level 10
The angular frequency ω is calculated as:
ω = 2πf = 2 * 3.14 * 245 = 1.5386 * 10³ rad/s ≈ 1.5 * 10³ rad/s
This continues the solution from number 15 on the previous page.
k = ω / v = (1.53 * 10³) / 300 = 5.1 m? ¹
A = 0.06 / 2 = 0.03m
New answer posted
4 months agoContributor-Level 10
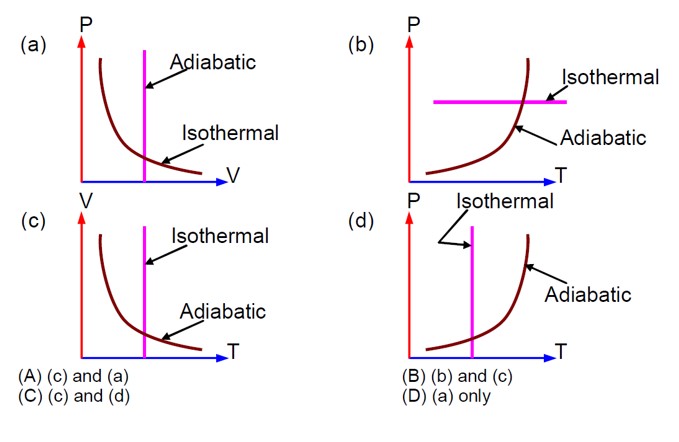
Pressure decreases with an increase in volume in both isothermal and adiabatic processes. In an adiabatic process, as volume decreases, pressure increases with the increase in temperature.
New answer posted
4 months agoContributor-Level 10
In an LCR series AC circuit, the phase difference φ between current and voltage is: φ = tan? ¹ (X? - X? ) / R)
If each vibrational mode contributes two degrees of freedom and the total degrees of freedom f = 3 + 3 + 4 = 10, then:
β = 1 + 2/f = 1 + 2/10 = 1.2
New answer posted
4 months agoContributor-Level 10
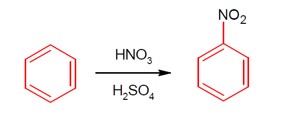
Moles of benzene = 3.9 g / 78 g/mol . This would produce (3.9 / 78) moles of nitrobenzene in 100% conversion.
Produced moles of nitrobenzene = 4.92 g / 123 g/mol .
% yield = [ (4.92 / 123) / (3.9 / 78) ] * 100 = [ (4.92 * 100 * 78) / (123 * 3.9) ] = 80.0%
Ans = 80
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers