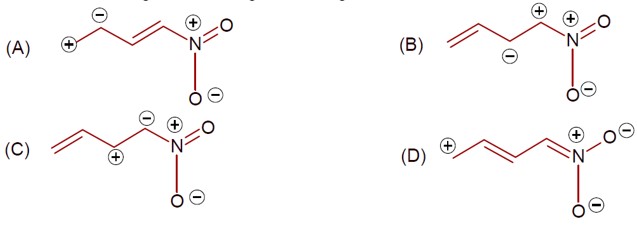Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 9
Oxidation states:
CrO? (+6), Fe?O? (+3), MnO? (+4), V?O? (+5), Cu?O (+1)
(a), (b), (c), (d), (e)
Order of oxidation numbers:
Cu?O < Fe?O? < MnO? < V?O? < CrO?. Hence correct order is; e < b < c < d < a
New answer posted
4 months agoContributor-Level 9
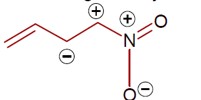
Same charge on adjacent atom is not stable. Hence the incorrect resonating structure is,
[Image of the incorrect resonance structure with adjacent positive charges]
New answer posted
4 months agoContributor-Level 10
q = +150 J
w = -200 J
∴ ΔU = q + w = 150 - 200 = -50 J
So, magnitude of ΔU is 50 J.
New answer posted
4 months agoContributor-Level 10
Due to small size Li have more polarizing power so most of the compound of Li are covalent.
New answer posted
4 months agoContributor-Level 10
Ba (OH)? ? Ba? ² + 2 (OH)?
Initial: 0.005M, 0, 0
Final: 0, 0.005, 0.010
[OH? ] = 0.01 M = 10? ² M
Kw = [H? ] [OH? ]
[H? ] or [H? O? ] = Kw/ [OH? ] = 10? ¹? /10? ² = 10? ¹² M
∴ [H? O? ] = 1 * 10? ¹² M
New answer posted
4 months agoContributor-Level 10
NaOH - Base
Be (OH)? - Amphoteric
Ca (OH)? - Base
B (OH)? - Acidic
Al (OH)? - Amphoteric
New answer posted
4 months agoContributor-Level 9
When positive charge increases, ionic radii decreases in isoelectronic ions
Al?³ < Mg? < Na? < K?
New answer posted
4 months agoContributor-Level 10
Δx . Δp = h/4π
Δx . mΔv = h/4π
Δv = 5 * 10? * 0.02/100 = 1000 m/s
∴ Δx = h/ (4πmΔv) = 6.63*10? ³? / (4*3.14*9.1*10? ³¹*1000)
= 5.8 * 10? m
= 58 * 10? m.
New answer posted
4 months agoContributor-Level 10
CH? + I? CH? I + HI
It is reversible due to reducing nature of HI but it can be irreversible by addition of oxidizing agents like, HNO? , HIO? , HgO, etc. which will oxidize HI into I?
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers