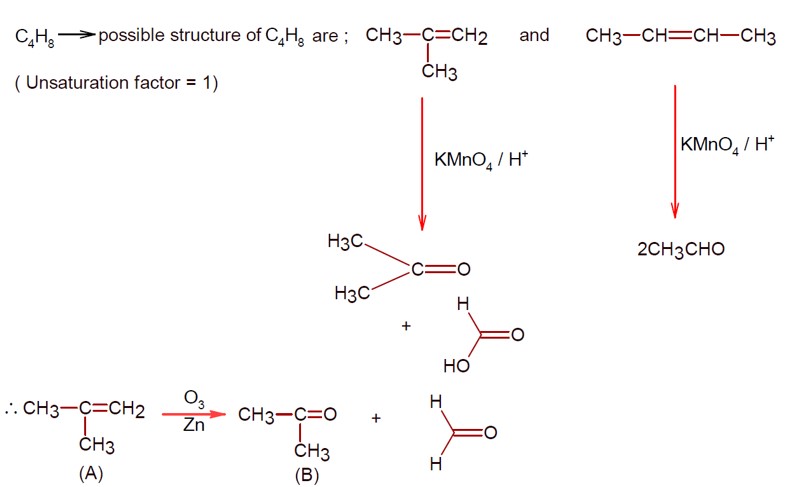
Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
P? = 300 kPa = 3 * 10? Pa = 3 bar
T? = 300 K
P? = 1.2 * 10? Pa = 12 * 10? Pa = 12 bar
T? =?
P? /T? = P? /T?
3/300 = 12/T?
T? = (12 * 300)/3 K = 1200 K
∴ T in °C = 1200 - 273 = 927°C
New answer posted
4 months agoContributor-Level 9
[SiCl?]?² is not known because six larger chloride ions can't be accomodated around Si? due to its small size.
New answer posted
4 months agoContributor-Level 10
CrO? ²? (Cr? ) → Cr? ³, Total oxidation number change = 3
MnO? (Mn? ) → Mn? ², Total oxidation number change = 5
Cr? O? ²? (Cr? ) → 2Cr? ³, Total oxidation number change = 6
C? O? ²? (C? ³) → 2CO? (C? ), Total oxidation number change = 2
New answer posted
4 months agoContributor-Level 9
B.D.E H-H < B.D.E D-D
B.D.E H-H = 435.9 kJ / mole
B.D.E D-D = 443.4 kJ / mole
E H ≈ E D - 7.5
New answer posted
4 months agoContributor-Level 10
Rotamers or conformers arises due to free rotation along σ - bond.
New answer posted
4 months agoContributor-Level 10
Rutherford atomic model can not explain hydrogen spectrum it is explained by Bohr's atomic model and from Bohr's atomic model, uncertainity principle can't be explained.
New answer posted
4 months agoContributor-Level 10
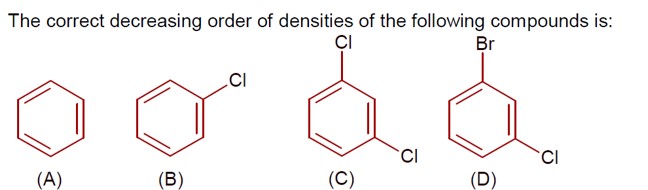
Density ∝ molar mass, as size of given molecules is nearly same
New answer posted
4 months agoContributor-Level 9
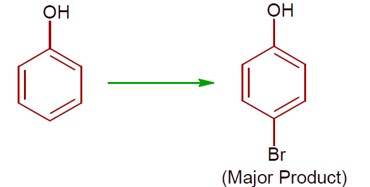
(a) Phenol with Br? / H? O gives 2,4,6-tribromophenol. (High ionisation due to polar solvent and high activation of ring)
(b) Phenol with Br? in CS? /CHCl? /FeBr? gives a mixture of o- and p-bromophenol, with p-bromophenol as the major product. (less ionisation due to non- polar solvent)
[Chemical reactions showing bromination of phenol under different conditions]
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers