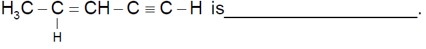Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 9
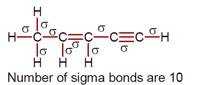
Number of sigma bonds are 10.
[Structure showing 10 sigma bonds in the molecule]
New answer posted
4 months agoContributor-Level 10
x² - |x| - 12 = 0
Case 1: x ≥ 0, |x| = x
x² - x - 12 = 0
(x-4) (x+3) = 0, x=4 (x=-3 is rejected)
Case 2: x < 0, |x| = -x
x² + x - 12 = 0
(x+4) (x-3) = 0, x=-4 (x=3 is rejected)
Two real solutions: 4 and -4.
New answer posted
4 months agoContributor-Level 10
x² - 4xy – 5y² = 0
Equation of pair of straight line bisectors is (x²-y²)/ (a-b) = xy/h
(x²-y²)/ (1- (-5) = xy/ (-2)
(x²-y²)/6 = xy/ (-2)
x²-y² = -3xy
x² + 3xy - y² = 0
New answer posted
4 months agoContributor-Level 10
H? O (l) → H? O (g)
ΔH° = ΔU° + ΔngRT
ΔH° - ΔU° = ΔngRT
= 1 * 8.31 * 373
= 3099.63 J/mol
= 30.9963 * 10² J/mol
≈ 31 * 10² J/mol
New answer posted
4 months agoContributor-Level 10
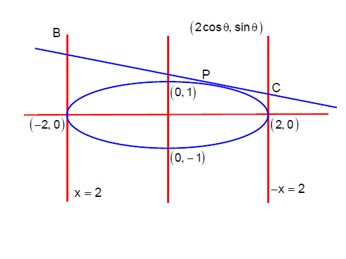
Equation of tangent of P (2cosθ, sinθ) is
(cosθ)x + (2sinθ)y = 4
Solving equation of tangent with equation of tangents at major axis ends, i.e. x = -2 and x = 2
For point 'B' (at x=-2):
-2cosθ + 2sinθ y = 4 ⇒ y = (2+cosθ)/sinθ
B (-2, (2+cosθ)/sinθ)
For point 'C' (at x=2):
2cosθ + 2sinθ y = 4 ⇒ y = (2-cosθ)/sinθ
C (2, (2-cosθ)/sinθ)
Now BC is the diameter of circle
Equation of circle: (x+2) (x-2) + (y - (2+cosθ)/sinθ) (y - (2-cosθ)/sinθ) = 0
x²-4 + y² - (4/sinθ)y + (4-cos²θ)/sin²θ = 0
Check if (√3, 0) satisfies this:
(√3)²-4 + 0 - 0 + (4-cos²θ)/sin²θ = -1 + (3+sin²θ)/sin²θ = -1 + 3/sin²θ + 1 = 3/sin²
New answer posted
4 months agoContributor-Level 10
Let volume of solution = x ml
So mass of solution = 1.2x
And mass of water = x gm
Mass of solute = 0.2x
Molality = (W_solute * 1000) / (M_solute * W_solvent) = (0.2x * 1000) / (40 * x) = 5 m
New answer posted
4 months agoContributor-Level 10
B.O. of CO = 3
B.O. of NO? = 3
Both are isoelectronic
So difference = 0
∴ x = 0
New answer posted
4 months agoContributor-Level 10
PCl? (g)? PCl? (g) + Cl? (g); Kc = 1.844
t=0: 3, 0, 0
equilibrium: 3-x, x
Kc = x² / (3-x) = 1.844
x² + 1.844x - 5.532 = 0
x = (-1.844 + √ (1.844² - 4 (1) (-5.532)/2 = (-1.844 + √25.528)/2 ≈ 1.604
At equilibrium number of moles of PCl? = (3 - 1.604) = 1.396 mol
= 1396 * 10? ³ mol
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers