Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Bohr's model played an important role in the development of quantum theory. It introduced the idea of quantised electron orbits. It disproved claims of classical mechanics, which predicted that electrons would spiral into the nucleus. Bohr proposed that electrons can exist only in specific, stable energy levels called stationary states. There would be transitions between these levels that would help Bohr explain the line spectra of hydrogen. That together linked the atomic structure with the concept of energy quantisation. Even though the model could not explain multi-electron atoms, it laid the foundation for modern quantum mechanics.
New answer posted
4 months agoContributor-Level 10
The existence of atomic spectra tells us that energy levels in atoms are quantised. When atoms absorb or emit light, they do so at specific wavelengths. They lead to line spectra instead of a continuous spectrum. Now, every line corresponds to an electron that transitions between fixed energy levels. This is to make the photon's energy equal to the difference between them. If energy levels were not discrete, the spectra would be continuous. So, the line spectra provide direct evidence that electrons in atoms occupy quantised energy states.
New answer posted
4 months agoContributor-Level 10
Metal of group 7, 8, & 9 dose not form interstitial hydride this is called hydride gap.
Mn → group - 7
Fe → group - 8
Co → group - 9
So, Cr will forms interstitial hydride.
New answer posted
4 months agoContributor-Level 10
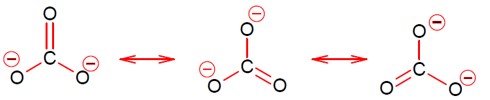
Conjugate base is highly stable.
Acidic strength ∝ stability of conjugate base.
So, 
New answer posted
4 months agoContributor-Level 10
O? = σ1s² σ1s² σ2s² σ2s² σ2pz² π2px² = π2py² π2px¹ = π2py¹
O? = σ1s² σ1s² σ2s² σ2s² σ2pz² π2px² = π2py² π2px¹
O? = σ1s² σ1s² σ2s² σ2s² σ2pz² π2px² = π2py² π2px² = π2py¹
O? ²? = σ1s² σ1s² σ2s² σ2s² σ2pz² π2px² = π2py² π2px² = π*2py²
B.O = (Bonding e? - Antibonding e? )/2
B.O of O? = (10 - 6)/2 = 2
B.O of O? = (10 - 5)/2 = 2.5
B.O of O? = (10 - 7)/2 = 1.5
B.O of O? ²? = (10 - 8)/2 = 1
New answer posted
4 months agoContributor-Level 10
Li? CO? decomposes easily on heating as;
Li? CO? - (Δ)-> Li? O + CO? ↑
NaHCO? is used in dry fire extinguishers.
K is most abundant element in cell fluid.
CsI is least soluble due to smaller hydration energy of Cs? & I?
New answer posted
4 months agoContributor-Level 10
Anions have larger radii than atoms. Also, higher the e/p ratio higher the ionic radii. So, N? ³ > O? ² > F?
New answer posted
4 months agoContributor-Level 10
CFC breakdown by visible light to give Cl radical which react with stratospheric ozone.
CFC, CF? Cl? - (hv)-> Cl• + •CF? Cl
Cl• (g) + O? → ClO• + O?
ClO• + O → Cl• + O?
Atmospheric ozone reacts with NO to give NO? and O?
O? + NO → NO? + O?
New answer posted
4 months agoContributor-Level 10
Bohr's model solved the instability problem by proving about stationary states. In such stats, the electrons move in fixed orbits. They do not emit energy. This contradicted classical electromagnetic theory of Maxwell, which says accelerating charges should emit radiation and collapse into the nucleus. Bohr simply assumed Maxwell's laws don't apply to these special orbits.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers