Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
The equation of the plane is given as x + y + z = 42. It is also mentioned that x³ + y³ + z³ = 3xyz.
From the identity, if x³ + y³ + z³ - 3xyz = 0, then x + y + z = 0 or x = y = z.
Given the expression:
3 + (x³ + y³ + z³ - 3xyz) / (xyz)²
Since x³ + y³ + z³ = 3xyz, the expression simplifies to:
3 + 0 = 3
New answer posted
4 months agoContributor-Level 10
The problem involves a function f (x) defined by a determinant:
f (x) = | sin²x 1+cos²x cos2x |
| 1+sin²x cos²x cos2x |
| sin²x cos²x sin2x |
Applying the row operation R? → R? - R? , we get:
f (x) = | -1 0 |
| 1+sin²x cos²x cos2x |
| sin²x cos²x sin2x |
Expanding the determinant along the first row:
f (x) = -1 (cos²x * sin2x - cos2x * cos²x) - 1 (1+sin²x)sin2x - sin²x * cos2x)
= -cos²x * sin2x + cos2x * cos²x - sin2x - sin²x * sin2x + sin²x * cos2x
= -sin2x (cos²x + sin²x) + cos2x (cos²x + sin²x) - sin2x
= -sin2x + cos2x - sin2x
= cos2x - 2sin2x
To find the maximum value of f (x), we use the form acosθ + bsinθ, where the m
New answer posted
4 months agoContributor-Level 10
The problem is to evaluate the integral:
I = ∫? ¹? [x] * e^ [x] / e^ (x-1) dx, where [x] denotes the greatest integer function.
The solution breaks the integral into a sum of integrals over unit intervals:
I = ∑? ∫? ¹ n * e? / e^ (x-1) dx
= ∑? n * e? ∫? ¹ e^ (1-x) dx
= ∑? n * e? [-e^ (1-x)] from n to n+1
= ∑? n * e? [-e? - (-e¹? )]
= ∑? n * e? (e¹? - e? )
= ∑? n * e? * e? (e - 1)
= (e - 1) ∑? n
= (e - 1) * (0 + 1 + 2 + . + 9)
= (e - 1) * (9 * 10 / 2)
= 45 (e - 1)
New answer posted
4 months agoContributor-Level 10
Yes, candidate who have secured 60% in Class 12 board exams can secure an admission into BSc courses offered by the NIU. The candidates must clear Class 12 in the PCB stream to be eligible for most BSc courses and then appear for the CUET entrance exam to qualify for admissions. The NIU BSc admissions are decided through the CUET entrance exam.
New answer posted
4 months agoContributor-Level 9
Fat-soluble vitamins are stored in our body for a relatively longer duration as compared to water-soluble vitamins.
- Vitamin B and C are water-soluble. (Thiamine is vitamin B1, while ascorbic acid is vitamin C).
- Vitamin A and vitamin D are fat-soluble, so they are stored in our body for a relatively longer duration.
New answer posted
4 months agoContributor-Level 9
The E° value for Ce? /Ce³? is +1.74 V, which suggests that Ce? is a strong oxidant, reverting to its common +3 oxidation state. So, Ce³? is more stable than Ce?
New answer posted
4 months agoContributor-Level 9
The size of the Bk³? ion is less than the Np³? ion because Berkelium (Bk) lies beyond Neptunium (Np) in the actinoid series, and the size variation here is because of the actinoid contraction.
New answer posted
4 months agoContributor-Level 9
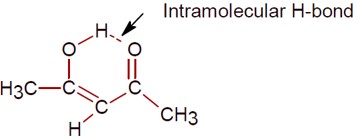
The enol form of acetone exists in less than 0.1% quantity, since its keto form is highly stable. But in the case of acetylacetone, the enol form is stabilized by intramolecular H-bonding, so its quantity increases to approximately 15%.
The intramolecular H-bond in the enol form of acetylacetone is shown.
New answer posted
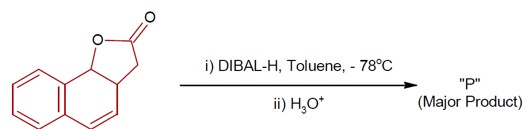
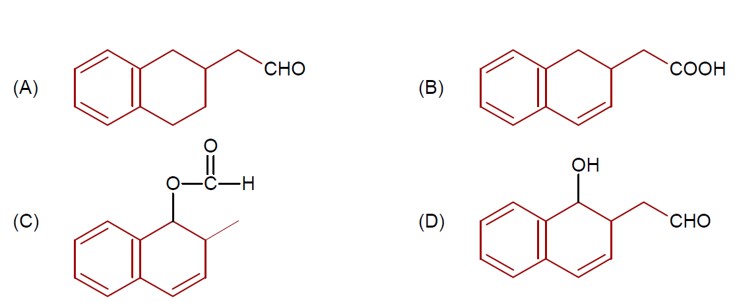
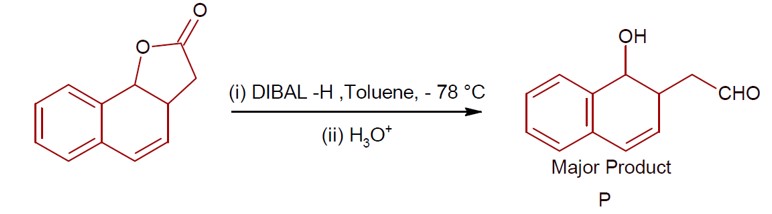
4 months agoContributor-Level 9
DIBAL-H at low temperature in a non-polar solvent, followed by hydrolysis, reduces esters to an aldehyde and an alcohol as a byproduct. The reaction shown is:
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers