Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Limit (n→∞) [[r] + [2r] + . + [nr]] / n²
We know that x - 1 < [x] x.
Summing from k=1 to n for [kr]:
Σ(kr - 1) < [kr] (kr)
rΣk - Σ1 < [kr] rk
r(n(n+1)/2) - n < [kr] r(n(n+1)/2)
Divide by n²:
(r/2)(1 + 1/n) - 1/n < ([kr])/n (r/2)(1 + 1/n)
As n → ∞, both the left and right sides approach r/2.
By the Squeeze Theorem, the limit is r/2.
New answer posted
4 months agoContributor-Level 10
cos(x)(3sin(x) + cos(x) + 3)dy = (1 + ysin(x)(3sin(x) + cos(x) + 3))dx
This seems mistyped. A more likely form is:
dy/dx - (sin(x)/(cos(x)))y = 1 / (cos(x)(3sin(x) + cos(x) + 3))
dy/dx - tan(x)y = sec(x) / (3sin(x) + cos(x) + 3)
The integrating factor (I.F.) is:
I.F. = e^∫(-tan(x))dx = e^(ln|cos(x)|) = cos(x).
Multiplying by I.F.:
d(y*cos(x))/dx = 1 / (3sin(x) + cos(x) + 3)
y*cos(x) = ∫ dx / (3sin(x) + cos(x) + 3)
Using Weierstrass substitution, let t = tan(x/2):
sin(x) = 2t/(1+t²), cos(x) = (1-t²)/(1+t²), dx = 2dt/(1+t²)
∫ (2dt/(1+t²)) / (3(2t/(1+t²)) + (1-t²)/(1+t²) + 3)
= ∫ 2dt / (6t + 1 - t² + 3 + 3t²) = ∫ 2dt / (2t² + 6t
New answer posted
4 months agoContributor-Level 9
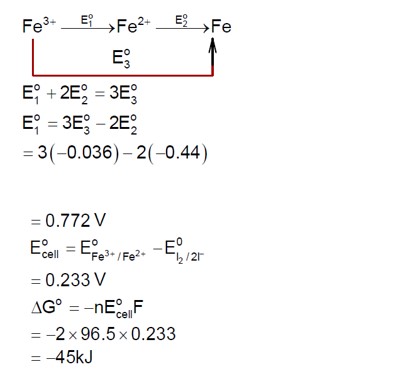
For the complex K? [Cr (oxalate)? ], the central metal ion is Cr³?
Electronic configuration of Cr (24) is [Ar] 4s¹3d?
Electronic configuration of Cr³? is [Ar] 4s?3d³.
The number of unpaired electrons in Cr³? is 3.
New answer posted
4 months agoContributor-Level 10
Given the function:
f(x) = { x(2 - sin(1/x)), if x ≠ 0
{ 0, if x = 0
For x < 0: f(x) = x(2 - sin(1/x))
For x > 0: f(x) = x(2 - sin(1/x))
The derivative f'(x) for x ≠ 0 is:
f'(x) = 1*(2 - sin(1/x)) + x*(-cos(1/x))*(-1/x²) = 2 - sin(1/x) + (1/x)cos(1/x)
The text calculates the derivative differently:
For x < 0: f'(x) = -2 + sin(1/x) - (1/x)cos(1/x)
For x > 0: f'(x) = 2 - sin(1/x) + (1/x)cos(1/x)
To check if f'(0) is defined, we would need to use the limit definition of the derivative at x=0. As x approaches 0, the term (1/x)cos(1/x) oscillates and does not approach a finite limit. Therefore, f'(0) is undefined.
New answer posted
4 months agoContributor-Level 9
Given K_f = 1.85 K kg mol? ¹ for a solution with molality of 2 m.
ΔT_f = I * K_f * m
3.885 = I * 1.85 * 2
The van't Hoff factor, I = 1.05.
i = 1 + (n-1)α. For an electrolyte dissociating into 2 ions, n=2.
1.05 = 1 + (2-1)α.
The degree of dissociation, α = 0.05 or 50 * 10? ³.
New answer posted
4 months agoContributor-Level 9
For an acidic buffer solution, pH = pKa + log ( [Base]/ [Acid]).
Given pH = 5.74 and pKa = 4.74.
5.74 = 4.74 + log ( [Base]/1).
1 = log ( [Base]).
[Base] = 10M.
New answer posted
4 months agoContributor-Level 9
For the reaction C? H? → C? H? + H? , calculate the enthalpy change (ΔH).
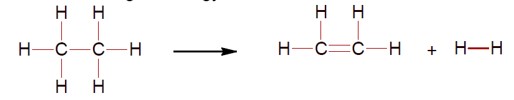
ΔH = [Bond energy (C-C) + 6 * Bond energy (C-H)] - [Bond energy (C=C) + 4 * Bond energy (C-H) + Bond energy (H-H)]
ΔH = 347 + 2 (414) - 611 - 436 = 128 kJ/mol.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers