Coordination Compounds
Get insights from 147 questions on Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: (A)
Linkage isomerism arises in a coordination compound containing ambidentate ligand.
A simple example is by complexes containing the thiocyanate ligand, NCS-which may bind through the nitrogen to give M-NCS or through sulphur to give M-SCN.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: A
CFSE for octahedral and tetrahedral complex is related as
Δt = Δ0
Where Δ0= CFSE for octahedral complex
Δ0= CFSE for tetrahedral complex
Δ0=1800 cm−1
Δt =49*18000=8000 cm−1
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: A
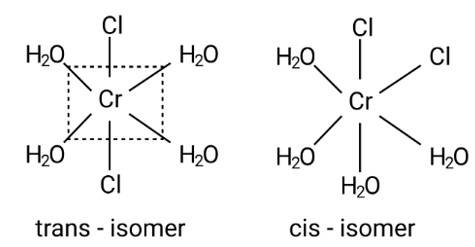
This is due to the complex's octahedral structure and (cis-trans) isomerism. Two Cl ligands are next to each other in trans isomerism. Although these isomers have the same structure, they are not identical.
Possible isomers are:
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: C
The chelate effect is when a chelating ligand coordinates the stabilisation of a molecule. Any ligand that binds to metal forms a ring with 5 or 6 members, which is the most stable.
Here, CO, CN, H2O is monodentate ligand [Fe (C2O4)3]3- is an oxalate ligand.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: A
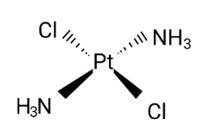
Diamminedichloridoplatinum (II) is also known as azane
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: D
When 1 mol [CrCl3 (H2O)3]6H2O is treated with excess of AgNO3, 3 mol of AgCl is produced, i.e., [CrCl3 (H2O)3]6H2Ois dissociated in aqueous solution and all three chloride comes in solution.
[Cr (H2O)6]Cl3→ [Cr (H2O)6]3+ + 3Cl-
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: B
When 0.1 mol CoCl3 (NH3)5 is treated with excess of AgNO3, In the given reaction [CoCl3 (NH3)5]Cl2
then electrolytic solution must contain
[CoCl3 (NH3)5]Cl2+ and two Cl- ions.
Hence, it is 1:2 electrolyte.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: A
Strong field ligands have five degenerate energy levels, which means they have more energy separation than weak field ligands.
Here, ΔE=
ΔE α
α
The wavelength decreases as the energy separation rises.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct options: B
The bigger the value of constant, the better will be the stability.
Here, has the highest value of logk which corresponds to the highest value of k.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Ambidentate ligands are ligands which have two donating sites. Coordinating compounds containing ambidentate ligands show linkage isomerism due to two different binding positions. Linkage isomerism have same ligand and geometry attached to a central metal ion by different donating sites
Examples:
(i)
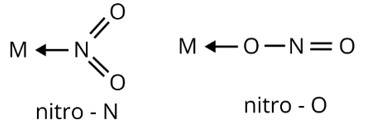
(ii) M←SCN
thiocyanato
M←NCS
isothiocyanato
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
