Coordination Compounds
Get insights from 147 questions on Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: Correct option: A
Toxic metal ions are removed by the chelating ligands. Chelate complexes tend to be more stable. When a solution of the chelating ligand is added to a solution containing toxic metals ligands chelates the metal ions by the formation of a stable complex.
Thus, EDT A (hexadentate ligand) forms a stable metal chelate and is used to remove toxic metal ions.
Both Assertion and Reason are correct and Reason is the correct for Assertion.
Hence, option A is correct.
New answer posted
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: Correct option: (i)
New answer posted
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: Correct option: (iv)
New answer posted
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: Correct option: (ii)
New answer posted
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: Correct option: (i)
New answer posted
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: Correct option: (ii)
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: C
Coordination compounds with the same composition but varying ligand-metal connectivity are known as linkage isomerism.
[Co (NH3)5 (NO2)]2+ , NO2 has two donor sites N and O
[Cr (NH3)5SCN]2+ , S and N are two donor sites.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct options: A, B and C
Option A, B, and C describe that ethane-1, 2-diamine is a neutral ligand due to its lack of charge, a bidentate ligand due to the presence of two donor sites on one nitrogen atom, and a chelating ligand due to its ability to chelate with metal.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: A and C
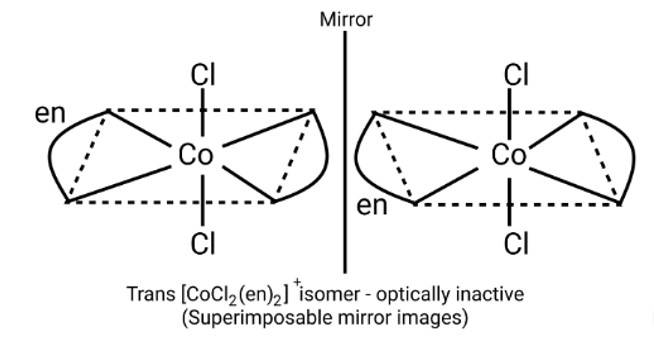
Optical compounds are highly imposable compounds that lack symmetry components. As a result, Option A and C are mirror reflections of each other.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: B and D
Heteroleptic is a compound that contains many ligands of various sorts. NH3 and
Cl as a ligand or also called as donor groups, is a heteroleptic complex.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
