Coordination Compounds
Get insights from 147 questions on Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
CN is strong field ligand
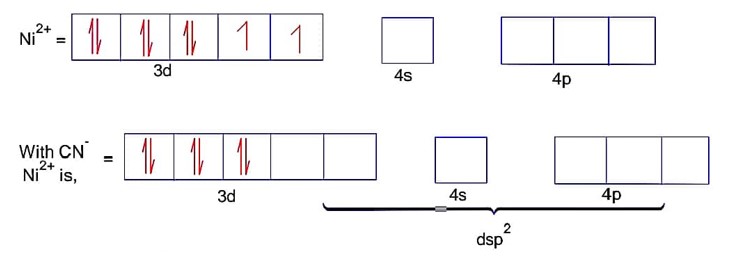
Here; is square planar and diamagnetic.
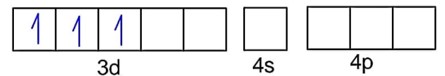
Ni = 4s23d8Co is strong field ligand.
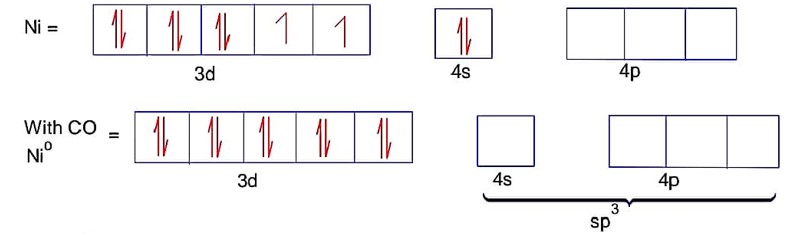
Here ; is tetrahedral and diamagnetic
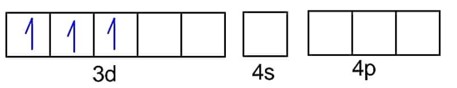
has 3d8 configuration while has 3d10 configuration.
New answer posted
6 months agoNew answer posted
6 months agoContributor-Level 10
A.
Fe3+ - 3d54s0
F- is weak field ligand, so no pairing of electrons :
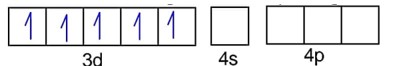
Number of unpaired electrons, n = 5
B.
CN- is strong field ligand, so pairing of electrons takes place.
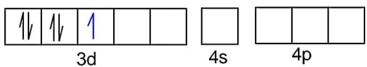
Number of unpaired electrons, n = 1
C.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: Correct option: D
[Fe (CN)6]3- ion shows magnetic moment corresponding to one unpaired electron. (CN) is a ligand with a strong field that couples electrons, resulting in hybridisation. d2sp3 and it has one unpaired electron, it has the magnetic moment of one paired electron.
μ= = 1 (1 + 2)
= 3 = 1.73BM
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: Correct option: B
From the Cis and Trans forms, geometrical isomerism forms compounds. All of the valences in the MX6 ligand are the same, resulting in an identical molecule. L is a distinct ligand in MX5L, and it can be replaced with any other valency in the structure to create an identical molecule; the molecule does not change. Because of the presence of symmetry, which is an essential prerequisite for revealing geometrical isomerism, complexes do not show geometrical isomerism.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: Correct option: A
The term "linkage isomerism" refers to two molecules that have similar ligands but differ in the donating site of the ligand. Ambidentate is made up of two different types of donor atoms.
Example [Fe (NO2) (H2O)5]Cl2. NO2 is Ambidentate ligand as Nand O2 are donors.
As a result, linkage isomerism occurs in coordinations with ambidentate ligands. Reason and Assertion are both correct.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: Correct option: B
[Cr (H2O)6]Cl2 and [Fe (H2O)6]Cl2
Due to the creation of a more stable complex ion after obtaining ion, [Cr (H2O)6]Cl2 and [Fe (H2O)6]Cl2 are reduced in nature. Both compounds have a coordination of 6 and so form an octahedral complex. The hybridisation state of is 3 and the oxidation state of is 2+. Hybridization is 3d4 and 3d6 is 2+. Both compounds have unpaired electrons and have weak field ligands. In both compounds, removing electrons is simple.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers