Coordination Compounds
Get insights from 147 questions on Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct options: A, B and C
A homoleptic complex is one that has only one species or group as a ligand.
Option A, B, and C each have only one ligand, but option D has two.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: B and C
[Co (H2O)6]2+ is increased when excess of HCl is added. Tetrahedral complexes have smaller crystal field splitting than octahedral complexes because Δt = Δ0
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: A and C
For answer at (A) Atomic number of Iron is 26 . In ferricyanide complex, iron is in oxidation state of 3+. The orbitals on the metal atom undergoes d2sp3 hybridizaion and hence the complex has octahedral shape.
Also it can be said that, [Fe (CN)6]3- has one unpaired electron which makes it weakiy paramagnetic. Therefore, option C is also correct.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: A and C
The outer octahedral complexes are weak ligands, number of pairing electrons and high spinning potential.
[MnCl6]3- - electronic configuration of Mn = 3d5,4s2. Shifting the valence electrons become
Mn3+ ,3d4 and number of unpaired electrons is 4.
[FeF6]3- - electronic configuration of Fe = 3d6,4s2. Shifting the valence electrons become
Fe3+ ,3d5 and the number of unpaired electrons is 5.
[CoF6]3 - electronic configuration of Co = 3d7,4s2 shifting the valence electrons become
Fe3+ ,3d6
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: A and C
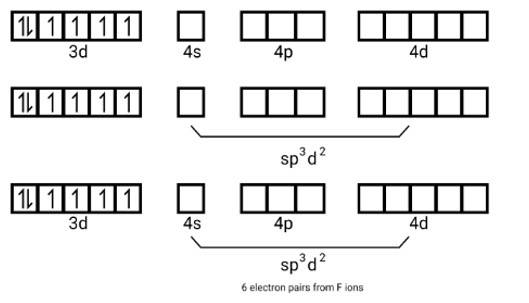
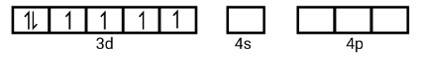
(i) Orbitals of Co3+ ion d2sp3 hybridised orbitals of Co3+ [Co (NH3)6]3+ (inner orbital or low spin complex)
No. of unpaired electron =0
Magnetic property = diamagnetic, due to absence of unpaired electrons
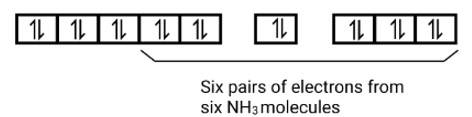
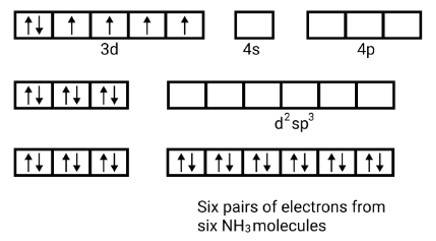
(ii) Electronic configuration is 3 d6 orbitals of Fe2+ ion:

6 electron pairs from CN− ions occupy the six hybrid d
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: C
[Pt (NH3)2Cl (NO2)] is a neutral molecule. Ligands are arranged alphabetically.
So, NH3 Diammine, chloride and the embedded linkage is nitrogen and last is the metal name.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: B
Hydrate isomerism is isomerism in which water is used as a solvent.
Coordination compounds with the same composition but varying ligand connectivity are known as linkage isomers.
Solvate isomerism has the same composition as free solvent but distinct solvent ligand molecules.
Except for the ligand that swaps places with the anion, ionisation isomers are identical isomers.
Coordination isomers are coordination compounds with distinct metal and ligand compositions.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: B
Ligands are neutral ions that form a coordination complex with the central metal atom.
NH4+ does not have a lone pair of electrons to give.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: A
A chelating agent binds to a single metal ion with two or more donor atoms. Oxaloto, Glycinato, and ethane-1,2-diamine all have two donor oxygen atoms. The gland Thiosulpato is ambidentate.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct option: D
Two or more compounds with the same formula but distinct structures and characteristics are known as isomers.
So, in this case compounds
[Co (SO4) (NH3)5]Br and [Co (SO4) (NH3)5]Cl show no isomerism
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers