Coordination Compounds
Get insights from 147 questions on Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The solution CrCl3.4H2O molar conductance shows that it contains one positive and one negative ion.
An ion is present outside the complex when silver chloride is treated with silver nitrate chloride. Outside the complex, there are two ions and one chloride, hence the name of the complex will be
[Co (H2O)4Cl2]Cl, Tetraaquadichloridocobalt (III)chloride.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Complexes with more number of ions show more conductivity.
[Co (NH3)3Cl3], [Co (NH3)4Cl2] < [Cr (NH3)5Cl]Cl2
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: The octahedral and tetrahedral splitting up of d orbitals is different.
Δt= ( )Δ0
Δt < 0
Δt= CFSE in tetrahedral field
Δ0= CFSE in octahedral field
Comparing CFSE with energy=
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: When light wavelengths from a specific part of the spectrum are absorbed by a substance, the result is a complimentary colour. When a complex absorbs a wavelength of light, it reflects a complementary colour. If a violent colour is absorbed, for example, yellow is conveyed. The CFSE value, often known as the colour definer for any complex, comes next. To determine the wavelength value in order to determine which colour absorbs the most energy.
Δe=
As λ has shorter wavelength.
Low spin complexes absorb shorter wavelengths, while high spin complexes absorb long
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: (i) 'A' is [Co (NH3)5SO4]Cl
'B' is [Co (NH3)5Cl]SO4
(ii) Type of isomerism is ionisation isomerism
(iii) IUPAC name of isomer 'A' is pentaaminesulphatocobalt (III)chloride
IUPAC name of isomer 'B' is pentaaminechlorocobalt (III)sulphate
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans:
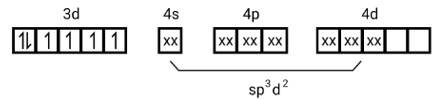
[Mn (CN)6]3−
Electronic configuration is Mn3+= [Ar]3 d4 hence box electronic structure
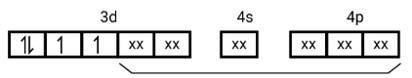
(i) Type of hybridisation d2sp3
(ii) Inner orbital complex
(iii) paramagnetic, due to presence of three unpaired electrons.
(iv) Spin only magnetic moment is calculated using the formula : n=2 in this case, we get spin only magnetic moment in BMas = = = 2.87BM
[Co (NH3)6]3+
Electronic configuration of Co3+= [Ar]3 d6
(i) Hyb As shown in the above box electronic structure the type of hybridisation is . d2sp3
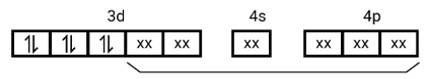
(ii) Inner orbital complex
(iii) Diamagnet
New answer posted
6 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
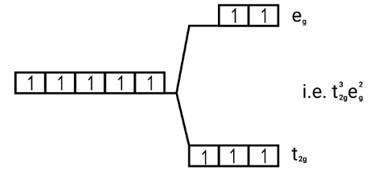
Ans: (i) Electronic cnfiguration: Co3+ =[Ar]3d6
Energy level diagram:
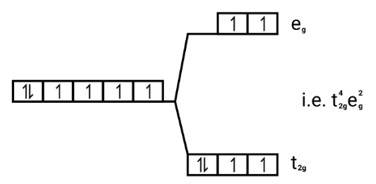
Magnetic moment:
Number of unpaired electrons (n)=4
Magnetic moment = μ= =
= = 4.9 BM
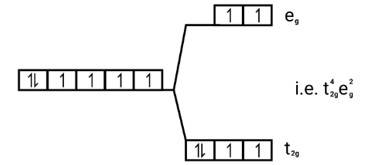
[Co(H2O)6]2+
Electronic cnfiguration: Co2+=[Ar]3 d7
Energy level diagram:
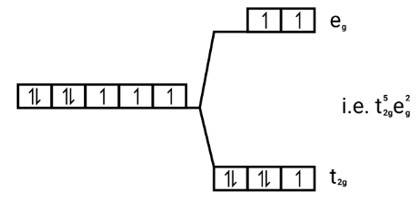
Magnetic moment: Since ,number of unpaired electrons (n)=3, therefore magnetic moment = = = 3.87BM
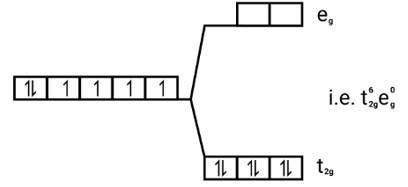
[Co(CN)6]3−
Electronic configuration: [Ar]Co3+=3 d6
Energy level diagram:
Ans: [FeF6]3−
Electronic configuration: Fe3+=[Ar]3 d5
Energy level d
New answer posted
8 months agoContributor-Level 10
The complex is an anion with chromium as central atom, 2 water molecules and 2 oxolate ions with -2 negative charge Balance overall charge as 0, we get oxidation state of Cr as:
X + 2 (0) + 2 (-2) = -1 X = + 3.
Name of compound: potassium diaquadioxolatochromate (III) trihydrate. Electronic configuration of Cr: 3d3, t2g3
New answer posted
8 months agoContributor-Level 10
(i) Overall charge balance:
X + 3 (-2) = -3 X = + 3
Oxidation state of Co is + 3.
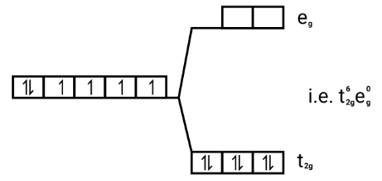
As there are 3 oxolate ion and being bidentate, coordination no. Of complex is 6. So it is octahedral complex.
d orbital occupation: t2g6eg0 (oxolate ion is weak field ligand, does not cause pairing of electron as the energy required for pairing of electron is more than CFSE).
(ii) Overall charge balance:
X + balance4 (-1) = -2 X = + 2
Oxidation state of Co is + 2.
As there are 4 fluoride ion, coordination no. Of complex is 4 i.e. Tetrahedral complex.
d orbital occupation: eg4t2g3 (fluoride ion is weak field ligand, does not cause pairing of electron as th
New answer posted
8 months agoContributor-Level 10
Compounds containing carbonyl
Ligands only are known as homoleptic carbonyl. Such types of compounds are formed by most of the transition metals. These metal carbonyls always have simple, well-defined structures. In metal carbonyls the metal - carbonyl bond possess both s and p.character. M-C-bond is sigma bond. It is formed by the donation of lone pair of electrons of the carbonyl carbon into the vacant orbital of the metal. The M-C pi bond is formed by the donation of a pair of electron from a filled d orbital of a metal into the vacant antibonding? orbital of carbon monoxide. Such type of metal to ligand bonding creates a synergic ef
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers