Coordination Compounds
Get insights from 147 questions on Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: In CuSO4⋅5H2O, water acts as ligand and causes crystal field splitting. This makes d - d transitions possible. On the other hand, in CuSO4, splitting is not possible due to the lack of ligand in the crystal field. As a result, no colour is visible.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Due to the presence of weak and strong field ligands in complexes, compounds with comparable geometry have distinct magnetic moments. The magnetic moment decreases when the CFSE increases, and vice versa.
Example: [Fe (CN)6]3- - Fe3+ ,3d5, CN- (strong field ligand, pairs electron)
[Fe (H2O)6]3- - Fe3+ ,3d5, H2O (weak field ligand, does not pair)
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: CFSE is higher when the complex contains strong field ligand. Thus, crystal field splitting energy increases in the order
[Cr (Cl)6]3−< [Cr (NH3)6]3+< [Cr (CN)6]3−.
Because according to spectrochemical series the order of field strength is
Cl−
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: CN- is a strong ligand and [Fe (CN)6]3- has one paired electron
H2O is a weak ligand and [Fe (H2O)6]3+ has five paired electrons.
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The ligands can be grouped in ascending order of increasing field intensity according to spectrochemical series.
[CoF6]3−, Co3+ → (d6)→ t42g e0g
[FE (CN)6]4−, Fe2+→ (d6)→ t62g e2g
[Cu (NH3)6]2+, Cu2+→ (d9)→ t62g e2g
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: In a tetrahedral complex, the d-orbit splits too little compared to an octahedral complex. As a result, orbital energy alone are insufficient to couple. Low spin tetrahedral complexes do not form as a result.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Weak field ligands, Electronic configuration of Co (III) is t42g e0g, has 4 paired electrons and it is paramagnetic.
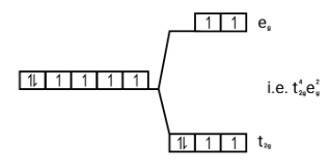
Strong field ligands, Electronic configuration of is Co (III) t62ge0g.

New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The existence of five unpaired electrons in the ion's mid-orbits Mn2+ equates to a magnetic moment of 5.92 BM. The number of hybridizations in this case is sp3. As a result, the magnetic moment value of the tetrahedral structure complex is 5.92 BM.
New answer posted
6 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
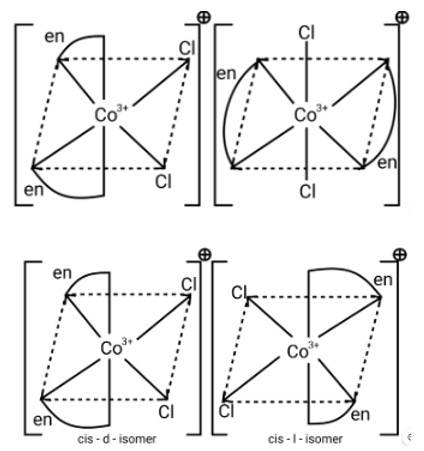
Ans: [M (AA)2X2]n + is a bidentate ligand and %X is a monodentate ligand. It is an example of octahedral geometry as well as an ion. There are cis and trans isomers of this ion. Trans isomers are symmetrically active, while cis isomers are optically active.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
