Coordination Compounds
Get insights from 147 questions on Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
Bonding in coordination compounds in terms of Werner's postulates is explained as:
(a) Metals can show two types of valencies which are Primary valency and Secondary
1. Primary Valency: Primary Valency shows Oxidation Primary valencies are ionizable.
2. Secondary Valency: Secondary Valency shows coordination These are non-ionizable.
(b) Both Primary and secondary valency of the metal are to be satisfied which is done by negative ions in case of primary valency and negative or neutral species in case of secondary
(c) Metals have a fixed number of secondary valencies/ Coordination number around the central atom, these secondary
New answer posted
8 months agoContributor-Level 10
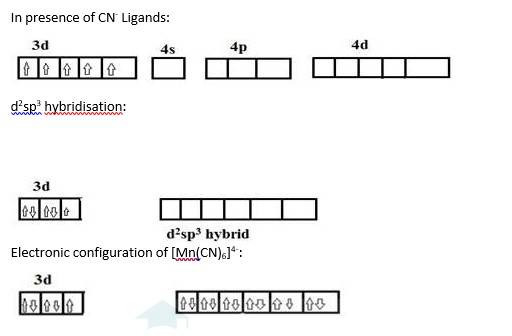
[Mn (H2O)6]+2 | [Mn (CN)6]4- |
Oxidation state of manganese: Overall charge balance: X + 6 (0) = 2 X = + 2 | Oxidation state of manganese: Overall charge balance: X + 6 (-1) = -4 X = + 2 |
Outer electronic configuration of Mn = d5 | Outer electronic configuration of Mn = d5 |
H2O is a weak field ligand so it does not cause pairing of the electron. Therefore Mn undergoes sp3d2 hybridization. Geometry is octahedral. Therefore the 5 unpaired electrons from the d orbital remain as it is. | CN is a strong field ligand so it causes pairing of the electron (5 electrons get paired to form 2 pairs and one unpaired electron). Therefore Mn undergoes d2sp3 hybridization. Geometry is octahedral. |
Mn in + 2 oxidation state:
New answer posted
8 months agoContributor-Level 10
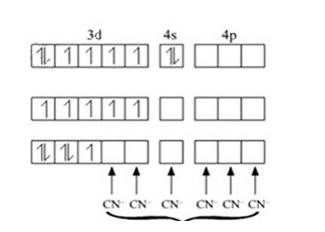
In Pt [ (CN)4]2- ion:
Overall charge balance:
X + 4 (-1) = -2 X = + 2.
The oxidation state of Pt is + 2.
Since CN- is a strong field ligand, it causes pairing of the unpaired electrons.
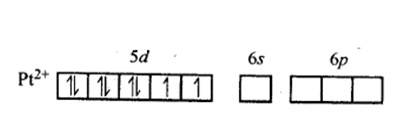
Therefore, now the 2 unpaired electrons from 5d orbital get paired and it undergoes dsp2 hybridisation. It forms square planar geometry. Since all the electrons are paired,
No. of unpaired electrons = 0.
New answer posted
8 months agoContributor-Level 10
[Co (NH3)6]3+ | [Ni (NH3)6]2+ |
Oxidation state of cobalt: Overall charge balance: X + 6 (0) = 3 X = + 3 | Oxidation state of nickel: Overall charge balance: X + 6 (0) = 2 X = + 2 |
Outer electronic configuration of cobalt = d6 | Outer electronic configuration of nickel = d8 |
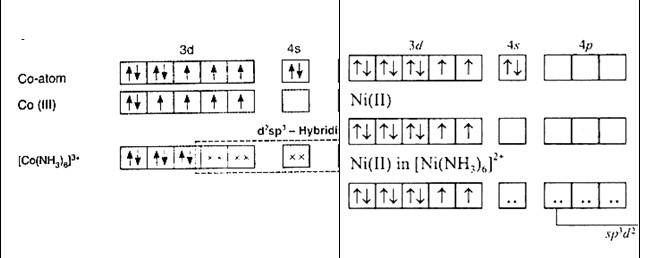
NH3 is a strong field ligand so it causes pairing of electron. Therefore cobalt undergoes d2sp3 hybridisation. As in the hybridisation d2 orbitals are used from the (n-1)d orbitals (inner orbitals as n = 4 being quantum number) . hence it is a inner orbital complex. | NH3 is a strong field ligand so it causes pairing of electron. Therefore, nickel undergoes sp3 d2 hybridisation. As in the hybridisation, d2 orbitals are used from the and orbitals (outer orbitals as n = 4 being quantum number). Hence, it is an outer orbital complex |
New answer posted
8 months agoContributor-Level 10
In [Fe (H2O)6]3+
Electronic configuration of Fe is: [Ar]3d64s2
[Ar] = 1s22s22p63s23p6
Electronic configuration of Fe+3 = [Ar]3d5
Outer electronic configuration of Fe+3 = 3d5
Overall charge balance:
X + 6 (0) = 3
X = + 3
In [Fe (CN)6]3-
Overall charge balance:
X + 6 (-1) = -3
X = + 3
In both the compounds Fe is in + 3 oxidation state.
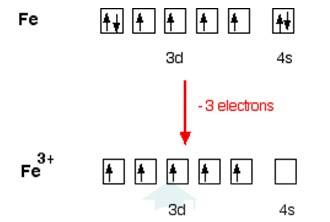
In case of [Fe (H2O)6]3+
H2O is weak field ligand so it does not pair the unpaired electron. Total no. of the unpaired electron, n =5, Spin only magnetic moment is given by:
μ = [n (n + 2)]1/2
μ = [5*7]1/2
μ = 5.916BM
In case of [Fe (CN)6]3-
CN- is a strong field ligand so it pairs up the electron.
Total no. of unpaired e
New answer posted
8 months agoContributor-Level 10
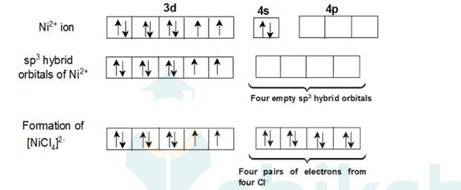
In [Ni (Cl)4]2- ion, Cl- is a weak field ligand so it will not pair the unpaired electrons of Ni+2 ion. Electronic configuration of Ni is: [Ar]3d84s2 where [Ar] = 1s22s22p63s23p6
Electronic configuration of Ni+2 = [Ar]3d8 Outer electronic configuration of Ni+2 = 3d8 Overall charge balance:
X + 4 (-1) = -2 X = + 2.
Therefore it undergoes sp3 hybridization. So it will have tetrahedral geometry.

Since there are 2 unpaired electrons in the d orbital so it is a paramagnetic compound. In [Ni (Co)4]:
Overall charge is neutral and oxidation state of Ni can be calculated as:
X + 4 (0) = 0
x = 0
Ni is in zero oxidation state.
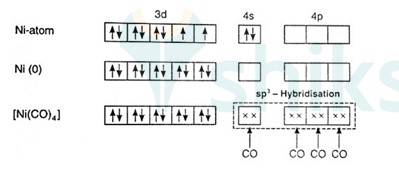
Co is a strong field ligand
New answer posted
8 months agoContributor-Level 10
According to the valence band theory, the central metal atom or ion under the influence of ligands can use its (n-1)d, ns, np (inner orbital complex) or ns, np, and (outer orbital complex)orbitals for hybridisation to form equivalent set of orbitals of definite geometry.
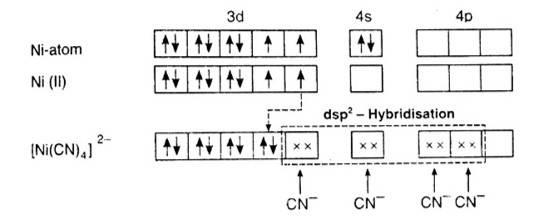
In [Ni (CN)4]2-, oxidation state of Ni can be calculated as :
Using overall charge balance as the whole ion has overall -2 charge:
x + 4 (-1) = -2 (? CN- has -1 negative charge) x = + 2
Ni is in + 2 oxidation state.
Electronic configuration of Ni is: [Ar]3d84s2 Where, [Ar] = 1s22s22p63s23p6
Electronic configuration of Ni+2 = [Ar]3d8 Outer electronic configuration of Ni+2 = 3
New answer posted
8 months agoContributor-Level 10
These compounds give different ions in aqueous solution. This can be tested by using AgNO3 solution and BaCl2
[Co (NH3)5Cl]SO4 (aq) + BaCl2 (aq) → BaSO4 (ppt)
[Co (NH3)5Cl]SO4 (aq) + AgNO3 (aq) → no reaction
[Co (NH3)5 (SO4)]Cl (aq) + BaCl2 (aq) → no reaction
[Co (NH3)5 (SO4)]Cl (aq) + AgNO3 (aq) → AgCl (ppt)
Hence, they give different precipitates with different solutions. Thus, they are ionisation isomers.
New answer posted
8 months agoContributor-Level 10
(i) K [Cr (H2O)2 (C2O4)2 exhibits geometrical isomerism (Cis and trans) and optical isomerism of cis and trans
(ii) Optical isomerism exhibiting mirror
(iii) Ionisation isomerism- [Co (NH3)5 (NO3)] (NO3) (NO2) and Linkage isomerism- [Co (NH3)5 (ONO)] (NO3)2
(iv) Geometrical isomers are seen in [Pt (NH3) (H2O)Cl2]
New answer posted
8 months agoContributor-Level 10
(i) Hexaminecobalt (III)chloride
(ii) Pentaaminechloridecobalt (III)chloride
(iii) Potassium hexacyanoferrate (III)
(iv) Potassium trioxalatoferrate (III)
(v) Potassium tetrachloridopalladate (II)
(vi) Diaminechloride (methylamine)platinum (II)chloride
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers