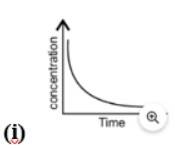
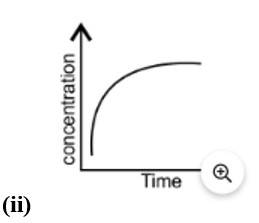
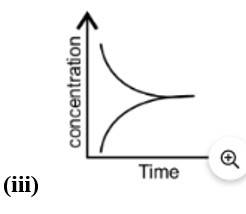
Equilibrium
Get insights from 241 questions on Equilibrium, answered by students, alumni, and experts. You may also ask and answer any question you like about Equilibrium
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) →c; (ii) → (d); (iii) → (a).
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) → (c); (ii) → (a); (iii) → (b).
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) → (b); (ii) → (e); (iii) → (c); (iv) → (d)
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) → (d); (ii) → (a); (iii) → (b).
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) → (d); (ii) → (c); (iii) → (b)
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) → (b); (ii) → (d); (iii) → (c); (iv) → (a).
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: options (i) and (iv)
Ice and water are in equilibrium only at particular temperature and pressure. For any pure substance at atmospheric pressure, the temperature at which the solid and liquid phases are at equilibrium is called the normal melting point or normal freezing point of the substance. For example, it is 4°C in case of water. The system here is in dynamic equilibrium.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans:, option (i), (iii) and (iv)
As, K increases with increase in temperature. K increases, it shows that the reaction must be endothermic. No. of molecules increases thus, there is increase in entropy.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iv)
Since in all the given reaction, the volume is to be kept constant and an inert gas argon is added which will do not take part in reaction. So, the equilibrium will remain unaffected in all the given cases.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (i)
If the equation is multiplied by 2, the equilibrium constant for the new equation is the square of K it means we must do the square of 5 that is 25. Then and on reversing the reaction the value of the equilibrium constant is inversed means the value of K comes out to be 1/25= 0.04.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers