Equilibrium
Get insights from 241 questions on Equilibrium, answered by students, alumni, and experts. You may also ask and answer any question you like about Equilibrium
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
vapour pressure of benzene at 20°C = 70 torr
= vapour pressure of toluene at 20°C = 20 torr
Mixture is equimolar, XB = 0.5 and XM = 0.5
Total vapour pressure (PT) = 70 * 0.5 + 20 * 0.5 = 45 torr
Mole fraction of benzene in vapour phase
= 0.777 = 77.7 * 10-2 torr
Ans. = 78 (the nearest integer)
New answer posted
6 months agoContributor-Level 10
for AgCl ppt1,
For Ag2CrO4ppt2,
Being lower concentration of [Ag+] in case of AgCl, it will precipitate first.
New answer posted
6 months agoContributor-Level 10
50 ml of 1 (M) HCl + 30 ml of 1 (M) NaOH
NaOH + HCl -> NaCl + H2O
30 * 1 mmol 50 * 1 mmol
0 mmol 20 mmol
x = 6021
Ans. = 6021
New answer posted
7 months agoContributor-Level 10
0.0504 M NH4Cl of 5ml => millimole of
0.0210 M NH3 of 2ml => millimole of NH3 = 0.0210 * 2
It is a basic buffer.
Total volume = 7ml
Ans. = 3
New answer posted
7 months agoContributor-Level 10
Statement I is true because methyl orange indicator has pH range (3.2 to 4.2) which is suitable for buffer produced by strong acid and weak base.
Statement II is false because phenolphthalein has pH range (8.4 to 10.2) and only suitable for equivalence point above than 7, In this case pH increased by the addition of NaOH on acetic acid.
New answer posted
7 months agoContributor-Level 10
Acid = M (OH)2 ® Salt + H2O
M.E of Acid = me of Base
Thus basicity of acid = 3
New answer posted
7 months agoContributor-Level 10
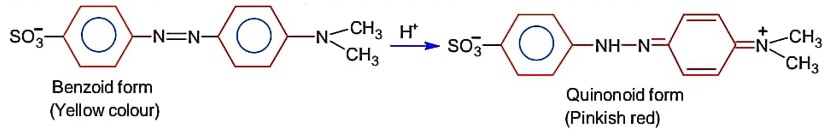
For an acid- base titration, Methly orange exist at end point as quinonoid form.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers