Equilibrium
Get insights from 241 questions on Equilibrium, answered by students, alumni, and experts. You may also ask and answer any question you like about Equilibrium
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoNew answer posted
7 months agoContributor-Level 10
Ka for C3H7COOH = 2 * 10-5
=5 – 0.3 = 4.7
pH of 0.2 (M) solution =
Ans 27
New answer posted
7 months agoContributor-Level 10
Eq. a(1 - α) aα (aα/2)
Moles moles moles
Total no. of moles at equilibrium
= nA + nB + nC
New answer posted
7 months agoContributor-Level 10
Metallic suphides i.e CdS are negatively charged colloidal solution.
Starch colloid is macro molecular colloidal solution.
Fe2O3. xH2O sol is positively charged colloidal solution.
Cheese is an example of gel.
New answer posted
7 months agoContributor-Level 10
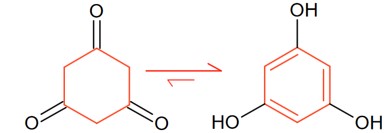
Aromaticity drives the highest enolic percentage of given structure:
New answer posted
7 months agoContributor-Level 10
Due to common ion effect solubility of AgCl will decreases in KCl, AgCl and AgNO3 but in deionized water, no common ion effect will takes place so maximum solubility.
New answer posted
7 months agoContributor-Level 10
Remaining volume of solution = 400 ml
Mass of HNO3 = 25.2 – 11.5 = 13.7
Molarity = = 0.54 M = 54 * 10-2 M
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers