Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
Ozone in the stratosphere is formed when UV radiation falls on the dioxygen (O2 ) molecules. The UV radiations split apart molecular oxygen into free oxygen (O) atoms, these oxygen atoms combine with molecular oxygen to form ozone. The reactions involved can be given as-
O2 (g) ![]() O (g) + O (g)
O (g) + O (g)
O (g) + O2 (g) +M![]() O3 (g) + M
O3 (g) + M
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
NO2 absorbs energy from sunlight and breaks up into nitric oxide and free oxygen atom and this can be shown as-
NO2 (g) NO (g)+O (g)
Here the oxygen atom produced is very reactive and combines with oxygen molecule in the air to give rise to ozone.
O (g) +O2 (g) + M → O3 (g) + M
Here 'M' is an inert gas like Nitrogen gas and ozone gets produced during the formation of smog.
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
The uncatalyzed oxidation of SO2 is a slow process. However, the presence of particulate matter in the polluted air catalyses the oxidation of SO2 to SO3. The reaction for the conversion can be given as-
2SO2 (g)+O2 (g)→2SO3 (g)
The reaction can be promoted by O3 or by H2O2.
SO2 (g)+O3 (g)→2SO3 (g)+O2 (g)
SO2 (g)+H2O2 (l)→H2SO4 (aq)
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
The symptoms which were observed in the village indicate that oxides of sulphur and nitrogen are released from the chimneys of the factory. These gases are obtained as the product of combustion of fossil fuels such as coal, gasoline etc. In an automobile engine, at high temperature, when fossil fuel is burnt, dinitrogen and dioxygen get combined to give significant quantities of nitric oxide and nitrogen dioxide. The chemical reactions can be given as-
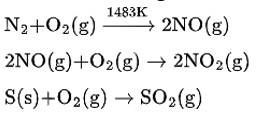
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
Due to the excessive growth of algae water contains a lot of phosphate due to the inflow of the fertilizers from the surroundings. The decomposition of algae leads to the bad smell and taste and even makes it unfit for drinking, bathing, washing, swimming, boating etc. and concentration of dissolved oxygen also decreases which may be harmful for aquatic life.
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
The amount of BOD of water is the amount of organic material present in water in terms of how much oxygen will be required to break it down biologically. Clean water has a BOD value less than 5 ppm. While highly polluted water has a BOD value of 17 ppm or more.
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
The oxygen reaches water through the atmosphere or by the process of photosynthesis carried out by green plants during daytime. But, during night as photosynthesis stops, the plants continue to respire, resulting in the reduction of dissolved oxygen. This dissolved oxygen is even used by microorganisms to oxidize organic matter. The sources of dissolved oxygen in water can be
(i) Photosynthesis by aquatic plants.
(ii) Due to the direct contact of the water surface with air.
(iii) Mechanical aeration.
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
The pollutants which get decomposed by bacteria like waste of vegetable and fruits, sewage, cow dung etc. are known as biodegradable pollutants. Non-biodegradable pollutants are those which do not get decomposed by bacteria e.g., mercury, polythene, aluminium, DDT etc.
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
The domestic wastes and organic compounds such as detergents provide nutrients to enhance the growth of algae and aquatic plants. These are decomposed by bacteria and give unpleasant odour. This process is being termed as eutrophication.
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
This is observed that the improper disposal of wastes is one of the major causes of environmental pollution. Whenever domestic waste is not properly managed, it goes to the sewers and is eaten by cattle. Non-biodegradable wastes like metals, glass etc., choke the sewers or gut of the ruminants also many a times polythene bags are swallowed by cattle resulting in their deaths. Similarly, when industrial waste is not properly managed it leads to the air, water, and soil pollution.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
