Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
In the stratosphere, chlorofluorocarbons get broken down by powerful UV radiations, releasing chlorine free radical. This can be shown as-
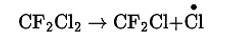
This chlorine radical then reacts with stratospheric ozone to form chlorine monoxide radicals and molecular oxygen. The reaction involved is-
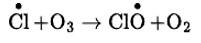
Reaction of chlorine monoxide radical with atomic oxygen produces more chlorine radicals and can be shown as-
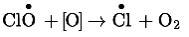
This shows that chlorine radicals are continuously regenerated and cause the breakdown of ozone. Hence, CFCs are transporting
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
The bacteria is held responsible for degrading biodegradable detergent feed on it and grows rapidly. While growing, they may use up all the oxygen dissolved in the water. The lack of oxygen kills all other forms of aquatic life such as fish and plants. Fertilizers contain phosphates as additives. The addition of phosphates in water further enhances algae growth. Such profuse growth of algae covers the water surface and reduces the oxygen concentration in water.
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
The atmosphere is divided into certain levels like troposphere, stratosphere, mesosphere, and thermosphere. The stratosphere consists of a considerable amount of ozone, and this protects us from the harmful ultraviolet radiations coming from the sun. These radiations cause skin cancer in humans. So, it is important to maintain the ozone shield. The main reason for ozone layer depletion is believed to be the release of chlorofluorocarbon compounds (CFCs), also known as Freon. UV radiation can cause genetic mutation and destroy crops, aquatic plants and ani
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
Rainwater has a pH of 5.6 due to the presence of H+ ions formed by the reaction of rainwater with carbon dioxide present in the atmosphere. The reaction can be shown as-
H2O (l)+CO2 (g)→H2CO3 (aq)
H2CO3 (aq)→H+ (aq)+HCO3− (aq)
If the pH of the rainwater falls below 5.6 then it is known as acid rain. SO2 and NO2 after oxidation and reaction with water are major contributors to the acid rain.
2SO2 (g )+ O2 (g) + 2H2O (l)→2H2SO4 (aq)
4NO2 (g)+O2 (g)+2H2O (l)→4HNO3− (aq)
New answer posted
5 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
Apart from carbon dioxide gas other greenhouse gases are methane, water vapour, oxides of sulphur, nitrous oxide, CFCs, and ozone. Methane is found to be produced naturally when vegetation is burnt, digested, or rotted in the absence of oxygen. Large amounts of methane are released in paddy fields, coal mines, from rotting garbage dumps and by fossil fuels. Chlorofluorocarbons (CFCs) are man-made industrial chemicals used in air conditioning etc. CFCs are also damaging the ozone layer. Nitrous oxide occurs naturally in the environment but is increasing day by d
New answer posted
5 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
Tetrachloroethene (Cl2C=CCl2 )was earlier used as solvent for dry cleaning. This compound contaminates the groundwater and is also suspected carcinogen. The process using this compound is now being replaced by a process where liquefied CO2 is used. Replacement of halogenated solvent by liquid carbon dioxide will result in less harm to the groundwater. These days, hydrogen peroxide is used for the purpose of bleaching clothes in the process of laundry, which gives better results and makes use of a lesser amount of water.
New answer posted
5 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
Pesticides can be defined as the synthetic toxic chemicals which are meant to kill pests. Most of the organic toxins are water insoluble and non- biodegradable in nature. These high persistent toxins are transferred from lower trophic level to the higher trophic level through the food chain. Over time, the concentration of toxins in higher animals reaches a level which merely causes serious metabolic and physiological disorders.
New answer posted
5 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
Since the earth's surface is heated by the sunlight, it radiates a part of this energy back to the space as longer Infrared wavelengths and some of the heat gets trapped by CFCs and water vapours present in the atmosphere. They absorb IR radiations and block a large portion of earth's emitted radiations. This radiation, absorbed, is partly remitted to the earth's surface. Therefore, the earth's surface gets heated up by the phenomenon called greenhouse effect. Thus, the greenhouse effect is defined as the heating up of the earth's surface due to trapping of infr
New answer posted
5 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
CO2 gas is confined to the troposphere only and it forms nearly 0.03 per cent by volume of the atmosphere. With the increased use of fossil fuels, a large amount of carbon dioxide gets released into the atmosphere. Excess of carbon dioxide in the air is removed by the green plants and this maintains an appropriate level of it in the atmosphere. Green plants require carbon dioxide for the process of photosynthesis and which in turn emits oxygen, thus maintaining the balance in nature. Deforestation and the burning of fossil fuels increase the level of carbon diox
New answer posted
5 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
(i) Many techniques are used to control or reduce the formation of photochemical smog.
(1) If we control the primary precursors of photochemical smog, such as nitrogen dioxide and hydrocarbons, the secondary precursors such as ozone and PAN of the photochemical smog will automatically get reduced.
(2) Mostly catalytic converters are used in automobiles to prevent the release of nitrogen oxide and hydrocarbons to the atmosphere.
(3) Certain plants e.g., Pinus, Juniparus, Quercus, Pyrus and Vitis metabolise nitrogen oxide and hence their plantation cou
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
