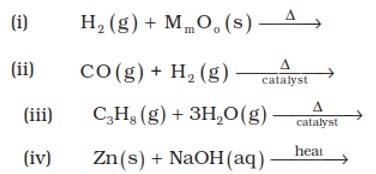Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
9.6. (i) 3H2?(g)+2MoO3? ? Mo2?O3?+3H2?O(l)
(ii) CO (g) + H2 (g) ? CH3OH
(iii) C3H8 (g) + 3H2O(g) ? 3CO + 7H2(g)
(iv) Zn (s) + NaOH (aq) ? Na2ZnO2(s) + H2(g)
New answer posted
6 months agoContributor-Level 10
9.5. In bulk, dihydrogen can be produced by electrolysis of acidified water using Platinum electrodes.
2H2O (l) → H2 (g) + O2 (g)
Electrolyte is added to increase the dissociation of water.
New answer posted
6 months agoContributor-Level 10
9.4. The production of dihydrogen in coal gasification can be increased by reacting CO present in syngas mixtures with steam in the presence of iron chromate as the catalyst. This is called the water-gas shift reaction. Synthesis gas or 'syngas' is produced from sewage, sawdust, scrap wood, newspapers etc. The process of producing 'syngas' from coal is called 'coal gasification'.
&nb
New answer posted
6 months agoContributor-Level 10
9.3. In diatomic form, the K-shell of hydrogen is complete (1s2) and so it is quite stable. That is why hydrogen occurs in a diatomic form rather than in a monoatomic form.
New answer posted
6 months agoContributor-Level 10
9.2.

Mass ratio of the isotopes = Protium: Deuterium: Tritium = 1: 2 :3
New answer posted
6 months agoContributor-Level 10
(i) Le Chatelier's principle states that the change in any factor such as temperature, pressure, concentration, etc. will cause the equilibrium to shift in such a direction so as to reduce or counteract the effect of the change.
(ii) (a) On adding Fe2O3 (s), the equilibrium will remain unaffected.
(b) By removing CO2 (g), the equilibrium will be shifted in the forward direction.
(c) By removing CO (g), the equilibrium will be shifted in the backward direction.
New answer posted
6 months agoContributor-Level 10
Whenever equilibrium is disturbed by change in the concentration, pressure or volume, the composition of the equilibrium mixture changes because the reaction quotient, Qc no longer equals the equilibrium constant, Kc. However, when a change in temperature occurs, the value of equilibrium constant, Kc is changed.
In general, the temperature dependence of the equilibrium constant depends on the signoff ΔH for the reaction.
- The equilibrium constant for an exothermic reaction (negative ΔH) decreases as the temperature increases.
- The equilibrium constant for an endothermic reaction (positive ΔH)increases as the temperature increases. Temper
New answer posted
6 months agoContributor-Level 10
If the volume is kept constant and an inert gas such as argon is added which does not take part in the reaction, the equilibrium remains undisturbed. It is because the addition of an inert gas at constant volume does not change the partial pressures or the molar concentrations of the substance involved in there action. The reaction quotient changes only if the added gas is a reactant or product involved in the reaction.
New answer posted
6 months agoContributor-Level 10
The intensity of the red colour becomes constant on attaining equilibrium. This equilibrium can be shifted in either forward or reverse directions depending on adding a reactant or a product. The equilibrium can be shifted in the opposite direction by adding reagents that remove Fe3+or SCN– ions. For example, oxalic acidH2C2O4), reacts with Fe3+ ions to form testable complex ion [Fe (C2O4)3]3–, thus decreasing the concentration of free Fe3+ (aq).In accordance with the Le Chatelier's principle, the concentration stress of removed Fe3+ is relieved by dissociation of [Fe (SCN)]2+ to replenish the Fe3+ions. Because the concentration of
New answer posted
6 months agoContributor-Level 10
This is because at lower pH, the concentration of the anion decreases due to its protonation. This in turn increase the solubility of the salt so that Ksp = Qsp.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers