Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
9.26. Water free from salts and minerals is called Demineralized water. Ion exchange method is used for this process. The ions present in the water bind to the positively or negatively charged sites on a resin when water is passed through the column packed with resin.
New answer posted
6 months agoContributor-Level 10
9.25. H2O2 as an oxidising agent:
2Fe2+ (aq) + 2H+ (aq) +H2O2 (aq) → 2Fe3+ (aq) + 2 H2O (l)
H2O2 as a reducing agent:
I2 (s) + H2O2 (aq) + 2OH– (aq) → 2I– (aq) + 2 H2O (l) + O2 (g)
New answer posted
6 months agoContributor-Level 10
9.24. Water is amphoteric in nature because it acts as an acid as well as a base.
Amphoteric nature of water is represented by following chemical reactions.
(1) Water as a base:
H2?O(l)+H2?S(g)?H3?O+(aq)+HS−(aq)
(2) Water as an acid:
H2?O(l) +NH3?(aq)?OH−+NH4+?
(3) Self ionization of water in which water simultaneously acts as acid and base.
2H2?O→H
New answer posted
6 months agoContributor-Level 10
9.23. Cation exchange resins have large organic molecule with SO3H group which are insoluble in water. Ion exchange resin (RSO3H) is changed to RNa on treatment with NaCl. The resin exchange Na+ ions with Ca2+ and Mg2+ ions present in hard water and make it soft.
2RNa (s) + M2+ (aq) ——> R2M (s) + 2Na+ (aq)
where, M = Mg, Ca.
The resins can be regenerated by adding aqueous NaCl solution.
New answer posted
6 months agoContributor-Level 10
9.22. Temporary hardness of water is due to the presence of bicarbonates of calcium and magnesium in water i.e., Ca (HCO3)2 and Mg (HCO3) in water. Permanent hardness of water is due to the presence of soluble chlorides and sulphates of calcium and magnesium i.e., CaCl2, CaSO4, MgCl2 and MgSO4.
New answer posted
6 months agoContributor-Level 10
9.21. Ice has crystalline structure which is highly ordered due to hydrogen bonding. It has hexagonal form at atmospheric pressure and cubic form at low temperature. Each O atom has tetrahedral geometry and is surrounded by 4 oxygen atoms each at a distance of 276 pm.
New answer posted
6 months agoContributor-Level 10
9.20. (i) PbS(s) +4H2O2(aq) → PbSO4(s) + 4H2O(l)
(ii) 2MnO4– (aq) +H2O2(aq) + 6H+(aq) → 2Mn (aq) + 8H2O(l) + 5O2(g)
(iii) CaO(s) + H2O(g) → Ca(OH)2(aq)
(iv) AlCl3(aq) + 3H2O(l) → Al(OH)3(s) + 3HCl (aq)
(v) Ca3N2(s) + H2O(l) → 3Ca(OH)2(aq) + 2NH3(aq)
(a) Hydrolysis reactions, (iii) (iv) and (v)
(b) Redox reactions (i) and (ii)
New answer posted
6 months agoContributor-Level 10
9.19. 2F2 (ag) + 2H2O (l)? O2 (g) + 4H+ (aq) + 4F (aq)
In this reaction water acts as a reducing agent and itself gets oxidised to O2 while F2 acts as an oxidising agent and hence itself reduced to F– ions.
New answer posted
6 months agoContributor-Level 10
9.18. Auto-protolysis means self-ionisation of water. It may be represented as
2H2O(l) + H2O(l) ? H3O+(aq) + OH-(aq)
Acid 1 Base 2 Acid 2 Base 1
Due to auto-protolysis nature of water, it can act as an acid as well as base, i.e. amphoteric in nature.
New answer posted
6 months agoContributor-Level 10
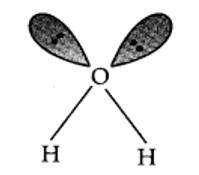
9.17. In water, O is sp3 hybridized. Due to stronger lone pair-lone pair repulsions than bond pair-bond pair repulsions, the HOH bond angle decreases from 109.5° to 104.5°. Thus, water molecule has a bent structure.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
