Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 9
Mole of Bromine = 0.08/80 = 10? ³ mole
Molar mass of compound = 0.172/M = 10? ³
M = 0.172/10? ³ = 172g
Molar mass of
New answer posted
4 months agoContributor-Level 9
(1) Pb (NO? )? → PbO + 2NO?
(2) NH? NO? → N? + 2H? O
(3) (NH? )? Cr? O? → N? + Cr? O? + H? O
(4) NaN? → 3N? + 2Na
New answer posted
4 months agoContributor-Level 10
16.00
X + Y? 2Z
Initial 1.0 1.5 0.5
At equ m 1-x 1.5-x 0.5+2x
equ m conc 0.75 1.25 1.0
0.5 + 2x = 1
x = 0.25
K = 1 / (0.75 * 1.25)
K = 16/15
x = 16.00
New answer posted
4 months agoContributor-Level 10
13.49
2 A (g) → A? (g)
Δn_g = 1 - 2
Δn_g = 1
ΔH = ΔU + Δn_gRT
ΔH = -20 * 10³ + (-1)8.31 * 298
ΔH = -22477.572 J mol? ¹
ΔG = ΔH - TΔS
ΔG = -22477.572 - (-30)298 = -22477.572 + 8940
ΔG = -13537.57 J mol? ¹
New answer posted
4 months agoContributor-Level 10
Boiling and clark's method (Ca (OH)? ) are used for removing temporary hardness. Whereas, calgon, sodium carbonate ion exchange method are used for removing permanent hardness.
New answer posted
4 months agoContributor-Level 10
Hydrogen peroxide has open book type structure. It is coloureless in free state
New answer posted
4 months agoContributor-Level 9
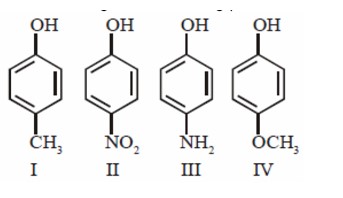
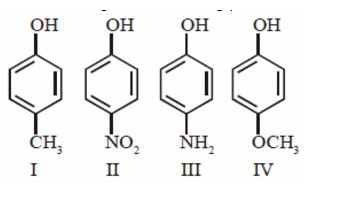
Isohexane and 3-Methylpentane having same molecular formula. Isohexane boil at and 3-Methyul pentane boils at . Both having low boiling point difference so fractional distillation is useful for separation and isohexane having low boiling point so comes out first.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers