Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
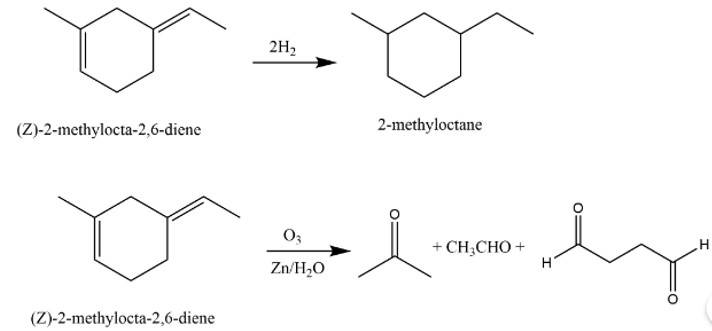
The structure of A is
The reaction involved are as follows:
New answer posted
5 months agoContributor-Level 10
An element can be identified by its atomic number only. Let us find the atomic number.
Let the number of protons = x
Number of neutrons = x + (x*31.7/100) = 1.371x
Now, Mass no. of element = no. of protons + no. neutrons
⇒ x + 1.317x = 81
⇒ x = 34.958
x ? 35
∴ No. of protons = 35, No. of neutrons = 81 – 35 =46
Atomic number of element (Z) = No. of protons = 35
The element with atomic number (Z) 35 is bromine (3579Br)
New answer posted
5 months agoContributor-Level 10
The composition of any atom can be represented by using the normal element symbol (X) with super-script on the left hand side as the atomic mass number (A) and subscript (Z) on the left hand side as the atomic number (i.e., AZX).
No two elements can have the same atomic number. However, the mass numbers have to be mentioned in order to identify the elements. Thus, symbols 7935Br and 79Br are accepted because atomic number of Br will remain 35 even if not mentioned.
For a given element, the number of protons is the same for the isotopes, whereas the mass number can be different for the given atomic number. Hence, correct pl
New answer posted
5 months agoContributor-Level 10
Heavy metals have a heavy nucleus and contain a large amount of positive change in their nucleus. By using heavy metals like gold and platinum in Rutherford's experiment, a large number of α-particles get deflected and experience a repulsion thus finding it hard for these α-particles to retrace their path.
If a thin foil of lighter atoms like aluminium were used in the Rutherford's experiment, the obstruction offered to the path of the fast moving α-particles would be comparatively quite less. As a result, the number of α-particles deflected will be quite less and the particles which are deflected back will be negligible.
New answer posted
5 months agoContributor-Level 10
Charge on oil droplet = – 1.282 x 10-18C
Charge on an electron = – 1.602 x 10-19C
Number of electrons = q /e = (– 1.282 x 10-18C) / (– 1.602 x 10-19C) = 8
New answer posted
5 months agoContributor-Level 10
Static electric charge (q) = 2.5 x 10-16 C
Charge on one electron (e) = 1.602 x 10-19 C
No. of electrons present = (2.5 x 10-16 C) / (1.602 x 10-19 C) = 1560
New answer posted
5 months agoContributor-Level 10
(a) The diameter of zinc atom is 2.6 Å =2.6*10−10m.
The radius of Zn atom is (2.6*10−10) / 2=1.3*10−10m=130*10−12m=130 pm.
(b) The number of Zn atoms present on 1.6 cm of length are 1.6 / (2.6*10−8) =6.154*107.
New answer posted
5 months agoContributor-Level 10
The length of the arrangement = 2.4 cm
Total number of carbon atoms present = 2 *108
Diameter of each C-atom = (2.4 cm) / (2 x 108) = 1.2 x 10-8 cm
Radius of each C-atom = ½ x 1.2 x 10-8 cm = 6.0 x 10-9 cm = 0.06 nm
New answer posted
5 months agoContributor-Level 10
Length of scale = 20 cm = 20 x 107 nm = 2 x 108 nm
Diameter of carbon atom = 0.15 nm
∴ Number of carbon atoms which can be placed side by side in a straight line across a length of a scale of length 20 cm = (2 x 108 nm) / (0.15 nm) = 1.33 x 109
New answer posted
5 months agoContributor-Level 10
The expression for the ionization energy atom,
En = (2.18 * 10-18 x Z2) / n2 J atom-1
For H atom, (Z = 1). So,
En =2.18 * 10-18 * (l)2 J atom-1 (given)
For He+ ion (Z = 2). So,
En =2.18 * 10-18 * (2)2 = 8.72 * 10-18 J atom-1 (one electron species)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
