Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The rate-determining step involved in the reaction is
CH3-CH=CH2 + HX → CH3-CH+-CH3 + X-
So the rate-determining step depends on the bond energy of HX i.e. higher the bond energy lesser would be reactivity.
Thus the order of reactivity of these halogen acids is
HI>HBr>HCl
New answer posted
5 months agoContributor-Level 10
Radius of orbit of H like species = (0.529 / Z) n2Å = (52.9 / Z) n2 pm
r1 = 1.3225 nm = 1322.5 pm = (52.9 / Z) n12
r2 = 211.6 pm = 211.6 pm = (52.9 / Z) n22
∴ r1 / r2 = 1322.5 / 211.6
=>n12 /n22 = 6.25
=> n1/n2= (6.25)1/2 = 2.5
=> n1 = 2.5 n2
=> 10 n1= 25 n2
=> 2 n1= 5 n2
If n1 = 2, then n2 = 5. That means transition occurs from 5th orbit to 2nd orbit. This means that the transition belongs to Balmer series.
Now, wave number? = (1.097 x 107 m-1) x (1/22 – 1/52) = 1.097 x 107 x 21/100 m-1 = 23.037 x 105 m-1
λ = 1/? = 1/ 23.037 x 105 m-1
= 434 x 10-9 m = 434 nm
This transition belongs to visible region of the spectrum of light.
New answer posted
5 months agoContributor-Level 10
Given, ν = (3.29 x 1015 Hz) (1/32 – 1/n2)
= (3.29 x 1015 Hz) (1/32 – 1/n2)
(3 x 108 ms-1) / (1.285 x 10-6 m) = (3.29 x 1015 Hz) (1/32 – 1/n2)
2.3346 x 1014 = (3.29 x 1015 Hz) (1/32 – 1/n2)
2.3346 / 32.9 = 1/32 – 1/n2
0.071 = 1/9 – 1/n2
1/n2 = 1/9 – 0.071= 0.111 – 0.071 = 0.04
n2 = 1/ 0.04 = 25
=> n = 5
For n = 5, Paschen series lies in infrared region of the spectrum.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The rotation around C-C single bond is possible but it is not completely free due to the torsional strain which is about 1-20 KJ mol-1.
New answer posted
5 months agoContributor-Level 10
λ = 150 pm, v = 1.5 x 107 m s-1
Kinetic energy, K.E. = ½ mv2 = ½ x 9.1 x 10-31 kg x (1.5 x 107 ms-1)2
= [ (9.1 x 1.5 x 1.5) / 2] x 10-31 +14
= 10.2375 x 10-17 J = 1.02375 x 10-16 J
K.E = hc/ λ = (6.626 x 10-34 kg m2 s-1) / (3 x 108 ms-1) / (1.5 x 10-10 m)
= [ (6.626 x 3) x 10-34+8+10] / 1.5
= 13.252 x 10-16 J
We know, E = W0 + K.E.
W0 = E – K.E. = (13.252 – 1.024) x 10-16 J
= 12.228 x 10-16 J
= 12.228 10-16 / 1.602 x 10-19
= 7.63 x 103 eV
New answer posted
5 months agoContributor-Level 10
λ = 256.7 nm = 256.7 x 10-9 m
K.E. = 0.35 eV
E = hc/ λ = (6.626 x 10-34Js) / (3 x 108 ms-1) / (256.7 x 10-9 m)
= (6.626 x 3 x 10-17) J/ 256.7
= (6.626 x 3 x 10-17) / (256.7 x 1.602 x 10-19) eV
E = 4.83 eV
The potential applied to silver gets converted into kinetic energy of the photoelectron.
So, Kinetic energy, K.E= 0.35 V
=> K.E= 0.35 eV
E = W0 + K.E.
=> W0 = E – K.E.
= 4.83 eV – 0.35 eV = 4.48 eV.
New answer posted
5 months agoContributor-Level 10
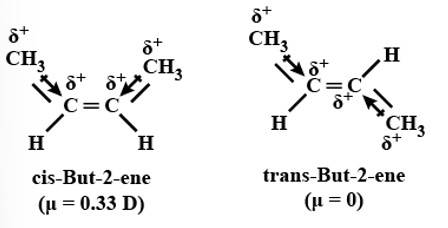
This is a short answer type question as classified in NCERT Exemplar
Yes, it will execute geometrical isomerism as the product formed can be either cis-2-butene or trans-2-butene.
New answer posted
5 months agoContributor-Level 10
Let the threshold wavelength be λ0 nm or λ0 * 10−9 m.
h (ν−ν0) = ½ mv2
hc (1/λ−1/λ0)= ½ mv2
hc [ (1/500*10−9) – (1/ λ0*10−9)] = ½ m (2.55*106)2 . (1)
Similarly,
hc [ (1/450*10−9) – (1/λ0? *10−9? )] = ½? m (4.35*106)2 . (2)
Similarly,
hc [ (1/400*10−9) – (1/λ0? * 10−9? )] = ½? m (5.2 * 106)2 . (3)
Divide equation (2) by (1),
[ (λ0 – 450) /450λ0] x – [500λ0/ (λ0 – 500)] = (4.35/ 2.55)2
(λ0 – 450)/ (λ0 – 500) = 2.61
λ0= 531 nm.
This is the threshold wavelength.
The value of the threshold wavelength is substituted in equation (3).
h *3*108 (1/400*10−9– 1/531*10−9)= ½ *9.1
New answer posted
5 months agoContributor-Level 10
W0 = 1.9 eV = 1.9 x 1.602 x 10-19 J
Threshold frequency, v0 = W0 / h = (1.9 x 1.602 x 10-19 J) / (6.626 x 10-34Js)
= 0.459 x 1015 s-1 = 4.59 x 1014 s-1
Threshold wavelength? 0 = c / v0 = (3 x 108 ms-1) / (4.59 x 1014 s-1)
= 0.6536 x 10-6 m = 653.6 nm? 654 nm
Kinetic energy, E = E0 + ½ mv2
(1/2 mv2) = E – E0 = hc [ (1/? ) – (1/? 0)]
= (6.626 x 10-34Js) x (3 x 108 ms-1) / (10-9) x [ (1/500) – (1/654)]
= 6.626 x 3 x 154 x 10-34+8+9) / (500 x 654)
= 9.36 x 10-20 J
Velocity, v = [ (2 x 9.36 x 10-20) / m]1/2
= [ (2 x 9.36 x 10-20) kg m2 s-2 / 9.1 x 10-31 kg]1/2
= (2.057 x 1011 m2s-2)1/2= (20.57 x 1010 m2s-2)1/2
= 4.5356 x 105 ms-1
New answer posted
5 months agoContributor-Level 10
λ1 = 589 nm = 589 x 10-9 m
ν1 = c / λ1 = (3 x 108 ms-1) / (589 x 10-9 m) = 5.0934 x 1014 s-1
λ2 = 589.6 nm = 589.6 x 10-9 m
ν2 = c / λ2 = (3 x 108 ms-1) / (589.6 x 10-9 m) = 5.0882 x 1014 s-1
ΔE = E1 – E2 = h [ν1 – ν2]
= (6.626 x 10-34Js) x [ (5.0934 x 1014 s-1) – (5.0882 x 1014 s-1)
= 3.31 x 10-22 J
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
