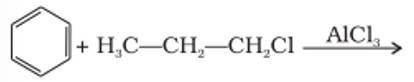Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
The electrons may be assigned to the following orbitals :
(i) 4d
(ii) 3d
(iii) 4p
(iv) 3d
(v) 3p
(vi) 4p.
The increasing order of energy is :
(v) < (ii) = (iv) < (vi) = (iii) < (i)
New answer posted
5 months agoContributor-Level 10

Since actual momentum is smaller than the uncertainty in measuring momentum, therefore, the momentum of electron cannot be defined
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
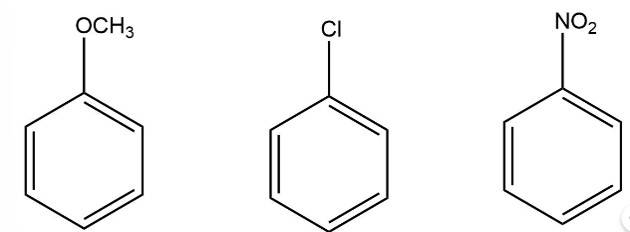
The electron donating group increases the reactivity in electrophilic substitution reaction whereas the electron withdrawing group decreases the reactivity in electrophilic substitution.
Thus, the order of reactivity
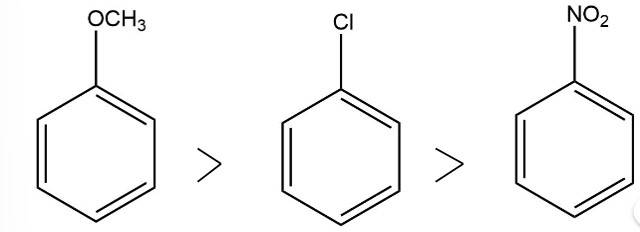
New answer posted
5 months agoContributor-Level 10
ν = 4.37 x 105 m s-1, m = 0.1 kg
As per de Brogile's equation,
λ= m/v = (6.626 x 10-34 kg m2 s-1) / (0.1 kg) x (4.37 x 105 m s-1)
=6.626/0.437 x 10-34-5 m
= 1.516 x 10-38 m
New question posted
5 months agoNew answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
New answer posted
5 months agoContributor-Level 10
ν = 2.19 x 106 m s-1
As per de Brogile's equation,
λ= m/v = (6.626 x 10-34 kg m2 s-1) / (9.1 x 10-31 kg) x (2.19 x 106 m s-1)
6.626/91 * 2.19= x 10-34+25 m = 0.33243 x 10-9 m = 332.43 pm
New answer posted
5 months agoContributor-Level 10
λ = 800 pm = 800 x 10-12 m
m = 1.675 x 10-27 kg
As per de Brogile's equation,
ν =h/mλ = (6.626 x 10-34 kg m2 s-1) / (1.675 x 10-27 kg) x (800 x 10-12 m)
=6.626/1.675 * 8 x 10-34+27+10
= 0.494 x 103 ms-1
= 494 ms-1
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The benzene here would undergo Friedel-Crafts alkylation reaction where carbocation is formed as an intermediate, thus secondary carbocation is formed as an intermediate due to its greater stability than that of the primary carbocation.
The final product obtained is
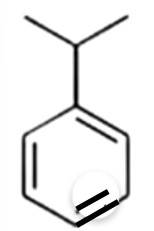
New answer posted
5 months agoContributor-Level 10
As per de Brogile's equation,
λ = h / mv = (6.626 x 10-34 kg m2 s-1) / (9.1 x 10-31 kg) x (1.6 x 106 ms-1)
= 0.455 x 10-9 m = 0.455 nm = 455 pm.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers