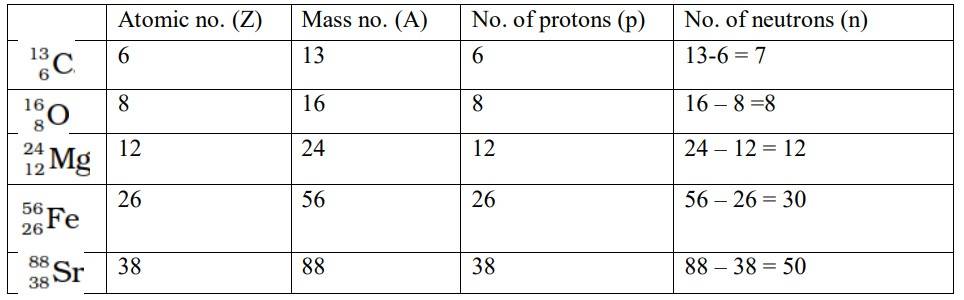
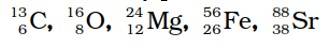Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
(i) Energy of photon (E) = hν, where h= Plank's const, ν= Frequency
h= 6.626 * 10-34 J s ; ν = 3 * 1015 Hz = 3 * 1015 s-1
∴ E = (6.626 * 10-34 J s) * (3 * 1015 s-1) = 1.986 * 1018 J
(ii) Energy of photon (E) = hν = hc/λ, where λ= wavelength
h = 6.626 * 10-34 J s ; c = 3 * 108 m s-1 λ= 0.50 Å = 0.5 * 10-10 m.
∴ E = (6.626 * 10-34 J s) * (3 * 108 ms-1) / 0.5 * 10-10 m = 3.98 x 10-15 J.
New question posted
5 months agoNew question posted
5 months agoNew answer posted
5 months agoContributor-Level 10
(i) One mole of methane (CH4) has = 6.022 * 1023 molecules
No. of electrons present in one molecule of CH4 = 6 + (4 x 1) = 10
No. of electrons present in 6.022 * 1023 molecules of CH4 = 6.022 * 1023 * 10
= 6.022 * 1024 electrons
(ii) (a)
Calculation of total number of carbon atoms
Gram atomic mass of carbon (C-14) = 14 g = 14 * 103 mg
14 g or 14 * 103 mg of carbon (C-14) has 6.022 * 1023 ato
New answer posted
5 months agoContributor-Level 10
43. A process which can take place of its own or initiate under some conditions. For example: Common salt dissolves in water of its own.
New answer posted
5 months agoContributor-Level 10
42. ?G = ?H – T?S
Where ?G = free energy change.
?H = enthalpy change.
?S = entropy change.
New answer posted
5 months agoContributor-Level 10
(i) Mass of an electron = 9.10939*10−31 kg = 9.1 * 10-28 g
⇒ No. of electrons in 9.1 * 10-28 g = 1 electron
Therefore, No. of electrons in 1 g = 1/9.1 * 10-28 = 1.098*1027
(ii) One mole of electrons = 6.022 * 1023 electrons
Mass of 1 electron = 9.1 * 10-31 kg
Mass of 6.022 * 1023 electrons = (9.1 * 10-31kg) * (6.022 * 1023) = 5.48 * 10-7 kg
Charge on one electron = 1.602 * 10-19 C
Charge on one mole electrons = 1.602 * 10-19 * 6.022 * 1023 = 9
New answer posted
5 months agoContributor-Level 10
41. Amount of heat required to vapourize one mole of a liquid at constant temperature and under standard pressure (1bar) is called its standard enthalpy of vapourization or molar enthalpy of vapourization, ΔvapH?.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers