Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Ans: Correct Option: (i)
(i)Mercury cells give a steady potential. The reason is that the ions are not involved in this reaction. The assertion is incorrect and the reason is correct so this option is incorrect
New answer posted
5 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Ans: Correct Option: (i)
(i) Molar conductivity of weak electrolytes increases on dilution. In addition to solution the degree of dissociation increases leading to an increase in the number of ions produced. Therefore, Λm shows a sharp increase.
Therefore, the assertion is correct, the reason is correct and the reason is a correct explanation for assertion.
(ii) Assertion is correct, the reason is correct and the reason is the correct explanation of assertion. Therefore, this option is incorrect
(iii)Assertion and true both are correct therefore
New answer posted
5 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Correct option: E
The order and molecularity may or may not be the same, assertion is false, but reason is right. The sum of the power of the reactants is the order of reaction, which may be easily determined. The total of the stoichiometric coefficient of the rate-determining elementary step determines molecularity
New answer posted
5 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Correct Option: (i)
Ans: (i) Conductivity is due to the movement of ions. The number of ions present per unit volume decides the conductivity. It decreases on dilution. The number of ions per unit volume decreases on dilution. So, the assertion is correct, the reason is correct and the reason is the correct explanation of the assertion. Therefore, this option is correct
(ii) Assertion is correct, the reason is correct and the reason is the correct explanation of assertion. Therefore, this option is incorrect
(iii) Assertion and true both are correct therefore this
New answer posted
5 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Correct option: B
Both assertion and reason are valid, but reason does not explain assertion because the order of reaction can be zero or even fractional because the order of reaction is proportional to the total of the power of the reactants.
New answer posted
5 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Ans: Correct Option: (iii)
(i)A chemical reaction is spontaneous when the Gibbs free energy is negative. This is possible when Ecell is positive. Ecathode < Eannode .Therefore, the assertion is correct and the reason is incorrect and the reason is not a correct explanation of assertion. Therefore, this option is incorrect
(ii) Ecathode < Eannode reaction is spontaneous when the Gibbs free energy is negative. This is possible when Ecell is positive. Ecathode < Eannode . Therefore, the assertion is correct and the reason is incorrect. Therefore, this option is co
New answer posted
5 months agoContributor-Level 10
This is a Assertion and Reason Type as classified in NCERT Exemplar
Ans: Correct Option: (iii)
(i)The electrode potential of Cu2+/Cu is negative. The electrode potential of 2H+/H2 is 0.00V. Therefore, the assertion is correct and the reason is incorrect and the reason is not a correct explanation of assertion. Therefore, this option is incorrect
(ii) E Cu2+/Cu is not negative so the reason is incorrect. Therefore, this option is incorrect
(iii) The electrode potential of Cu2+/Cu is negative. The electrode potential of 2H+/H2 is 0.00V. Therefore, the assertion is correct and the reason is incorrect. Therefore, this opt
New answer posted
5 months agoContributor-Level 10
This is a Matching Type Question as classified in NCERT Exemplar
(i)- (b) ; (ii)- (a) ; (iii)- (d); (iv)- (c)
The rate rule was discovered experimentally and can be used to predict the reaction rate and reactant concentrations.
The rate constant is the proportionality constant that describes the relationship between the molar concentration of the reactants and the rate of a chemical reaction.
The rate constant (k) of a reaction is proportional to its temperature, i.e., for a given reaction at a given temperature, the rate constant (k) is constant.
The order of a reaction is a quantity that has been empirically determined. As a result
New answer posted
5 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) F2 is a non-metal which is the best oxidizing agent since the standard reduction potential of F2 is 2.87V.
(ii)Liis a metal and the strongest reducing agent because the standard reduction potential of Li is −3.05V.
(iii)Au3+ is a metal ion which is an oxidizing agent as standard reduction potential of Au3+ is 1.40V.
(iv) Br−is an anion that can be oxidized by Au3+ as standard reduction potential of Au3+ is 1.40V which is more than that of Br− which is E Br2/Br−0=1.09V.
(v)Auis an unreactive metal
(vi)Li+ is a metal ion having the least value
New answer posted
5 months agoContributor-Level 10
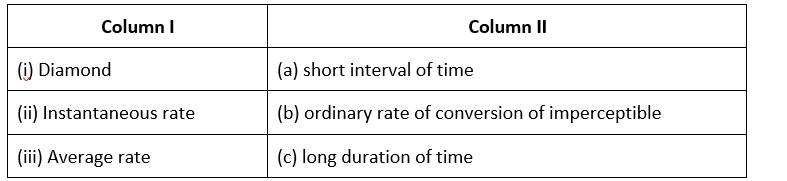
This is a Matching Type Question as classified in NCERT Exemplar
(i)- (b) ; (ii)- (c) ; (iii)- (a)
The rate of conversion in diamond is normally imperceptible.
Long-term rate at a moment's notice
The average rate is only for a short time.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers