Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (i, iii)
The conductivity in an ionic solution is due to the ions in the solution. It depends on the following factors;
(a)Temperature: On an increase in temperature, the molar conductivity of ionic solutions increases.
(b)Concentration of Electrolytes: An increase in the concentration of electrolytes decreases the molar conductivity as the number of ions per unit volume decreases.
Therefore, options (i, iii) are correct
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: B, C and D
Catalysts reduce the activation energy of a process and give an alternate path by reducing or raising the activation energy between reactants and products, altering the reaction's enthalpy change. As a result, B, C, and D statements are the proper responses
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (i, ii)
Conductance is the reciprocal of resistance and conductivity is the reciprocal of resistivity. It can be written as follows
k =
R=
=
k =
k =
k = G*
k =
G* is cell constant.
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (i, ii)
In the electrolysis process of CuSO4 the following reactions occur at the half cell which is as follows:
At the cathode, the reaction goes this way,
Cu2++2e−→Cu (s)
At the anode, the reaction goes this way,
Cu (s)→Cu2++2e−
Here copper will be deposited at the cathode and copper will dissolve at the anode.
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and B
Arrhenius equation k = Ae –
When the temperature rises, value falls and –
As a result, e- increases, and it is certain that k and T increases as well. As a result, Option A is proven.
Catalyst increases the rate of reaction by lowering the activation energy. Thus option B is also correct.
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
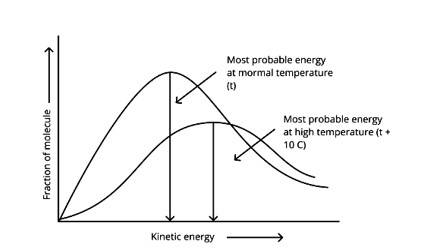
Correct options: A and D
According to the graph, the area under the cure must not vary as the temperature rises.T2 > T1
The energy level of T2 is higher than T1.
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (i, iii)
Electrolysis of copper sulfate solution is as follows
CuSO4 ? Cu2+ + SO42−
H2O? H+ + OH−
At the cathode, the reaction goes this way,
Cu2+ + 2e− → Cu; E? Cell = 0.34V
H2O− → H2 E? Cell = 0.00V
At the anode, the reaction goes this way,
2SO42- + 2e−→S2O82− E? Cell =1.96V
2H2O→O2 + 4H+ + 4e− E? Cell =1.23V
The reaction will lower the value of E? Cell is preferred at anode so the second reaction is feasible.
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (i, iii)
Kohlrausch law of states that limiting molar conductivity of any salt species is equal to the sum of the limiting molar conductivity of cations and anions of the electrolyte
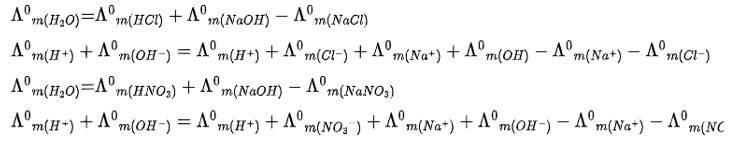
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and C
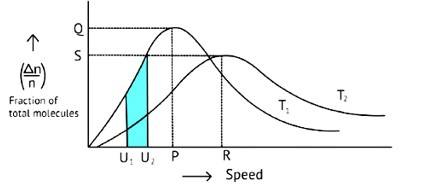
The Maxwell Boltzmann distribution, often known as the Gibbs distribution, is a measure that expresses the likelihood of a system being in each state as a function of the energy of that state.
Look at the graph.
T2 > T1
The fraction of molecules falls as the temperature rises because the area under the curve shrinks.
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Correct Option: Option (i, ii)
Conductivity is due to the movement of ions in the solution. The conductivity of ions depends on the following factors:
(i) nature of electrolyte added
(ii) size of ion produced
(iii) concentration of electrolyte
(iv) nature of the solvent
(v) temperature
Distance between electrodes does not affect the conductivity of an electrolytic solution.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers