Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
The pace of any reaction is determined by the concentration of the reactants, the rate of any reaction generally declines during the reaction. As the reaction progresses, the reactant concentration declines, and the product concentration rises.
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
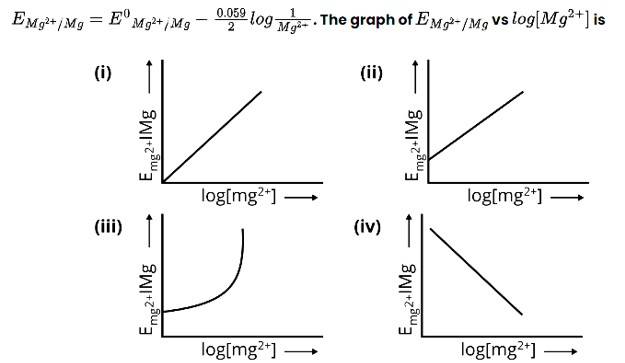
Ans: Correct Option: (iii)
(i) For measurement of electrode potential standard conditions must be met which is 1 bar pressure and 1M concentration which is not here so this option is incorrect.
(ii) For measurement of electrodes potential standard conditions must be met which is 1 bar pressure and 1M concentration which is not here so this option is incorrect.
(iii) When copper electrode is connected to SHE it acts as cathode and its standard electrode potential can be measured as follows
E E R- E L
For calculationn of standard potential of a given cell it shoul
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Acetic acid is a weak electrolyte and the number of ions on dilution increases due to an increase in the degree of dissociation. In the case of strong electrolytes the number of ions remains the same but interionic attraction decreases.
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The cell reaction of a lead storage battery is as follows;
Pb + PbO2 + 2H2SO4→2H2SO4 + 2H2O
The density of electrolytes changes because water is formed and sulphuric acid is consumed as a product during discharge of the battery
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
The chance of more than three molecules meeting at the same time is extremely minimal, reactions with molecularity more than three are extremely rare. Simultaneous collisions decrease as the number grows, the probability is very low, and more than three reactions with molecularity are uncommon.
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Primary batteries contain a limited amount of reactants and once consumed are discharged. Secondary batteries take a long time to recharge. Fuel cells run continuously as long as reactants are supplied to them and products are consumed
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Question as classified in NCERT Exemplar
The activation energy for fuel combustion reactions is very large, and because every substance or element requires an ignition temperature to function and excite to a higher temperature, oxygen in air does not combust to fire.
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The reactions taking place in the Daniel cell is as follows;
Zn+ + Cu2+ → Zn2+ + Cu
The number of electrons involved in two. The Nernst equation is as follows;
Ecell = E cell - log
From the above equation, we conclude that increasing the concentration of Zn2+, Ecell will decrease.
New answer posted
5 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The given cell reaction consists of two half cell reactions represented below as follows;
At anode: Cu→Cu2++ 2e−
At cathode: Cl2 + 2e− →2Cl−
Here oxidation of copper and reduction of chlorine is taking place
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers