Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
4.10 (i) 3NO (g)? N2O (g)
Rate = k [NO]2
Here rate law is dependent on concentration of NO only. And power raised to concentration of NO is 2. Hence order of reaction is 2.
To find the dimensions of rate constant (K)- We know the rate of chemical reaction is measured in (concentration / time). So, the unit of K will be
(concentration) / (time) (concentration)2
i.e. [concentration]-1 [ time]-1 . If concentration is in mol L-1 and time is in sec. then dimensions of k will be : mol-1 L sec-1
(ii) H2O2 (aq) + 3I- (aq) + 2H+? 2 H2O (l) + I3-
Rate = k [H2O2] [I-]
Here rate law is dependent on concentration of H2O2 and I-. And power ra
New answer posted
6 months agoContributor-Level 10
There are four different types of ligands present in the complex. So, by fixing the position of 2 ligands we get 2 geometrical isomers and by changing the position of fixed isomer we get one more geometrical isomer.
Since it is tetrahedral complex so it should be optically active . But however it has not been possible to resolve optically active d and l forms of such a complex due to its complicated nature.
New answer posted
6 months agoContributor-Level 10
Two types of ligand : chloride ion is unidentate ligand and -en (ethylenediamine) is bidentate ligand with two sites of attachment. The complexes in which two symmetrical bidentate chelating ligands AA and two monodentate ligands a, are coordinated to central metal atom M, exhibit the phenomenon of optical isomerism and can be resolved into their optical isomers.
An example of this type of complexes is given as shows both geometrical as well as optical isomerism. Its cis form is unsymmetrical, while the trans form is symmetrical because it contains a plane of symmetry. Hence, optical isomerism is shown by cis form.
New answer posted
6 months agoContributor-Level 10
Optical isomers are the one which rotate the plane polarized light by a certain angle when passed through them and are mirror images of each other, also called as stereoisomer.
(i) C2O4 2- is a bidentate ligand so it can attach to central atom at two sites, forming a chelate ring. In the complexes of this type, three symmetrical bidentate chelating ligands AA are coordinated to the central metal atom M. Such complexes do not possess any element of symmetry and are optically active. Moreover, these complexes can be resolved into optical
(ii) In this complex Cl is an unidentate ligand with one site of attachment whereas -en (ethyle
New answer posted
6 months agoContributor-Level 10
(i) No geometrical isomer is possible for [Cr (C2O4)3]3– because the ligand C2O 2- is bidentate ligand (which have two sites of attachment to the central atom) and also in the coordination sphere it is the only ligand bond to it.
(ii) In the coordination sphere of [Co (NH3)3Cl3] there are two types of ligands present i.e NH3 and Cl- . Coordination number is There are 2 isomers possible for the complex:
Facial: In this isomer one type of ligand say NH3 forms the face of the square bipyramidal (triangular) structure.
Meridional: In this isomer one type of ligand are along the central axis of the pyramidal structure.
New answer posted
6 months agoContributor-Level 10
4.9 Given-
Energy of activation, Ea= 209.5 kJ mol–1
Ea in joules = 209.5 * 1000 = 209500 J mol–1
Temperature, T = 581K
Gas constant, R = 8.314 J K-1 mol-1
Using the Arrhenius equation,
k = Ae-Ea/RT
In this equation, the term e-Ea/RT represents the number of molecules which have kinetic energy greater than the activation energy, Ea.
∴The number of molecules = e-Ea/RT → Equation 1. Substituting all the known values in equation 1, we get,
= e-209500/8.314X581
=e-43.3708
Finding the value of anti ln (43.3708), we get, 1.47 * 10-19
The fraction of molecules of reactants having energy equal to or greater than activation energy is 1.47 * 10-19
New answer posted
6 months ago9.18 List various types of isomerism possible for coordination compounds, giving an example of each.
Contributor-Level 10
Isomers are the compounds which have same chemical formula but different arrangement of atoms in space. There are principle two types of isomerism:
(i) Stereo isomerism
(ii) Geometrical isomerism
(iii) Optical isomerism
(iv) Structural isomerism
(v) Ionisation isomerism
(vi) Linkage isomerism
(vii) Coordination isomerism
(viii) Solvate isomerism
Geometrical isomerism comes into existence by the different spatial arrangement of groups around the central metal atom. Similar groups may either be arranged on the same side or on opposite sides of the central metal atom. This gives rise to two types of isomers called cis and trans isomers. Wh
New answer posted
6 months agoContributor-Level 10
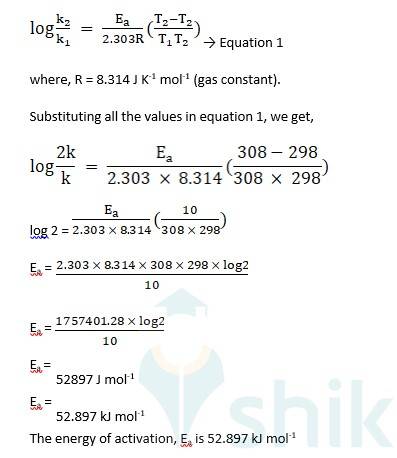
4.8 Given-
Initial temperature, T1 = 298 K
Final temperature, T2 = 298 K + 10 K = 308 K
Knowing that the rate constant of a chemical reaction normally increases with increase in temperature, we assume that,
Initial value of rate constant, k1 = k
Final value of rate constant, k2 = 2k
Using Arrhenius equation,
New answer posted
6 months agoContributor-Level 10
(i) Starting with cation, the complex ion contains six ammonia molecules with cobalt in + 3 oxidation The name of compound: [Co (NH3)6]Cl3 is hexaamminecobalt (III) chloride.
(ii) The complex ion is cation, so there are 2 ammonia molecules, one chloride ion and methyammine molecule qith platinum in + 2 state. Going in alphabetical order, the name of compound: [Pt (NH3)2Cl (NH2CH3)]Cl is diamminechloridomethylammineplatinum (II)
(iii) It is a complex cation with six water molecules and titanium atom in + 3 state. The name of the compound: [Ti (H2O)6]3+ is hexaaquatitanium (III) ion
(iv) The complex ion is cation with
New answer posted
6 months agoContributor-Level 10
4.7 The rate constant of a chemical reaction normally increases with increase in temperature. It is observed that for a chemical reaction with rise in temperature by 10°C, the rate constant is nearly doubled and this temperature dependence of the rate of a chemical reaction can be accurately explained by Arrhenius equation,
k = A
Thus, the rate constant of a chemical reaction normally increases with increase in temperature.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
