Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
16.10
The use of chemicals for therapeutic effect is called chemotherapy.
For example: the use of chemicals in the diagnosis, prevention, and treatment of diseases like cancer etc.
New answer posted
6 months agoContributor-Level 10
5% solution means 5g of cane sugar is present in 100g of solution
Freezing point of solution = 271k
Freezing point of pure water = 273.15k
Molar mass of cane sugar (C12H22O11) = 12 * 12 + 1 * 22 + 16 * 11 = 342g
Moles of cane sugar = mass/molar mass = 5/342
⇒ n = 0.0146mol
Molality of solution = moles of solute/mass of solvent (in kg)
⇒ M = 0.0146/0.095
⇒ Molality = 0.154M
Depression in freezing point = ΔTf = 273.15-271 = 2.15k
Applying the formula: ΔTf = Kf * M
Where
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ Kf = 2.15/0.154
⇒ Kf = 13.96k kg mol-1
Second condition: mass of glucose = 5g
Mola
New answer posted
6 months agoContributor-Level 10
16.10
A medicine has the tendency to bind to more than one receptor site. Thus, a medicine may be toxic for some receptor sites. Further, in most cases, medicines cause harmful effects when taken in higher doses than recommended. As a result, medicines may be poisonous in such cases. Hence, medicines should not be taken without consulting doctors.
New answer posted
6 months agoContributor-Level 10
Given: mass of solute = 30g
Let the molar mass of solute be x g and vapour pressure of pure water at 298k be P1 ?
Mass of water(solvent) = 90g
Molar mass of water = H2O = 1 * 2 + 16 = 18g
Moles of water = mass of water/molar mass
⇒ n = 90/18 moles
⇒ n = 5moles
Molar fraction of solute,
x2 = moles of solute / moles of solute + moles of octane
x2 = (30/x) / (30/x) + 5
x2 = 30 / 30+5x
Vapour pressure of solution (p1) = 2.8kpa
Applying the formula:

According to second condition when we add 18g of water to solution vapour pressure becomes 2.9kpa
Moles of water = mass/molar mass
⇒ n = 90 + 18/18
⇒ n = 6moles
Molar fraction of solute,
x2 = mo
New answer posted
6 months agoContributor-Level 10
16.9
The macromolecules that are chosen as drug targets are carbohydrates, lipids, proteins, and nucleic acids.
New answer posted
6 months agoContributor-Level 10
16.8
In medicinal chemistry, drug targets refer to the specific key molecules involved in certain metabolic pathways that result in specific diseases. Carbohydrates, proteins, lipids, and nucleic acids are examples of drug targets.
Drugs are chemical agents designed to inhibit these target molecules by binding with the active sites of the key molecules.
New answer posted
6 months agoContributor-Level 10
16.6
The basis on which drugs are classified in different ways is : - of drugs and the reasons for classification are as follows:
(i) On the basis of pharmacological effect:
This classification provides doctors the whole range of drugs available for the treatment of a particular type of problem. Hence, such a classification is very useful to doctors.
(ii) On the basis of drug action:
This classification is based on the action of a drug on a particular biochemical process.
(iii) On the basis of chemical structure:
This classification provides the range of drugs sharing common structural features and often having similar pharmaco
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
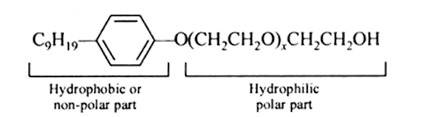
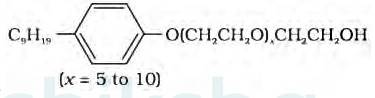
The functional group present in the molecule is:
1. Ether
2. Primary Alcoholic group
Alcohol group can recognize by the presence of –OH group present in the given organic compound and on the other hand, the ether group can be identified by the presence of R-O-R bonds where R represents the alkyl group present in the compound.
New question posted
6 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers