Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoNew answer posted
6 months agoContributor-Level 10
16.22
Artificial sweetening agents refer to those compounds which impart sweet taste to any food product but at the same, they do not add any calories to the body. Example: Aspartame, Alitame, Saccharin etc.
New answer posted
6 months agoContributor-Level 10
The Ptotal for the values given in the graph is found out and plotted in the graph.
ptotal (mm Hg) | 632.8 | 603.0 | 579.5 | 562.1 | 580.4 | 599.5 | 615.3 | 641.8 |

It can be observed from the graph that the plot for the p total of the solution curves downwards. Therefore, the solution shows negative deviation from the ideal behaviour.
New answer posted
6 months agoContributor-Level 10
16.21
At elevated temperatures, aspartame is unstable and break down to give a tasteless compound because of which aspartame is limited to cold foods and drinks.
Aspartame is methyl ester of the dipeptide obtained from phenylalanine and aspartic acid. It is about 180 times as sweet as cane sugar. The structure of aspartame is as follows:
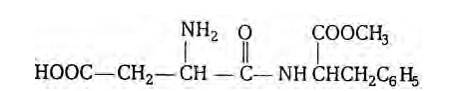
New answer posted
6 months agoContributor-Level 10
Given-
Mass of liquid A, WA = 100g, Molar mass, MA = 140 g mol-1
Mass of liquid B, WB = 1000 g, Molar mass, MB = 180 g mol-1
Using the formula below calculate the no. of moles in liquid A and B.
Number of moles = Mass / Molar Mass
Number of moles of liquid A, MA = 100/140 = 0.714 mol-1
Number of moles of liquid B, MB = 1000/ 180 = 5.556 mol-1
Using the formula,
mole fraction of a liquid = No. of moles of the liquid / total no of moles
we calculate the mole fraction of liquids A and B.
→ Mole fraction of A,
xA = 0.714 / (0.714 + 5.556)
∴ xA = 0.114
→ Mole fraction of B,
xB = 1- xA = 1 - 0.114
∴ xB = 0.886
Vapour pressure of pure liquid B, Po
New answer posted
6 months agoContributor-Level 10
16.20
Food preservatives are chemicals that prevent food from spoilage due to microbial growth. Table salt, sugar, vegetable oil, sodium benzoate (C6H3COONa), and salts of propanoic acids are some common examples of food preservatives.
New answer posted
6 months agoContributor-Level 10
Given-
Henry's law constant KH = 4.27X 105 mm Hg,
p = 760mm Hg,
Using Henry's law,

Using the formula of lowering vapour pressure,

Thus, the solubility of methane in benzene is 0.023 moles
New answer posted
6 months agoContributor-Level 10
Given- Vapour pressure of water,
PA0 = 17.535 mm Hg
WB= 25 g of glucose
WA = 450g of water
Molar mass of water, H2O = 1 + 1 + 16 = 18 g mol-1
Molar mass of glucose, C6H12O6 = (12*6) + (1*12) + (16*6) = 180 g mol-1
Using Raoult's law for solution of non-volatile solute,
PA0 - PA / PA0 = xB? Equation 1
where xB is the mole fraction of the solute
xB = WB/MB X MB/WB
=25/180 X 18 / 450
xB = 1/180
Substituting the value of xB in equation 1, we get,

Thus, the vapour pressure of water at 293 K at the given conditions is 17.437 mm Hg
New answer posted
6 months agoContributor-Level 10
Given- w1 = 500g
W2 = 19.5g
Kf = 1.86 K kg mol-1
Molar mass of CH2FCOOH = 12 + 2 + 19 + 12 + 16 + 16 + 1
= 78 g mol-1
The depression in freezing point is calculated by,

→ (where, m is the molality)
= 1.86 X 19.5 / 78 X 1000/500
= 1.86 X 19.5 / 78 X 2
=0.93
∴ Δtf (calculated) = 0.93
To find out the vant Hoff's factor, we use the formula,
i = observed Δtf / calculated Δtf
i = 1.0 (given) / 0.93
∴ i= 1.07
CH2FCOOH → CH2FCOO- + H +
To find out the degree of dissociation α, we use

Thus, the vant Hoff's factor is 1.07 an the dissociation constant is 2.634x10-3
New answer posted
6 months agoContributor-Level 10
16.19
Iodine is a powerful antiseptic. It is employed as tincture of iodine which is an alcohol-water solution containing 2-3 percent of iodine. Iodine is widely used in healing and treatment of wounds as it kills all the microbes present in the wounded region.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers




