Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
Mass of CH3CH2CHClCOOH = 10 g
Mass of water = 250g
Ka = 1.4 * 10–3,
Kf = 1.86 K kg mol–1
Molar mass of CH3CH2CHClCOOH = 12 + 3 + 12 + 2 + 12 + 1 + 35.5 + 2 + 16 + 16 + 1
= 122.5 g mol–1
Number of moles of solute = Mass of Solute / Molar Mass
→ No. of moles = 10g / 122.5 g/mol
∴ No. of moles = 8.6 X 10–2 mol
Now, Molality is given as,
M = Number of moles of solute / kg of solvent
M= 8.6 X 10–2 X 1000 g/mol / 250 g
M = 0.3264 kg/mol
CH3CH2CHClCOOH = CH3CH2CHClCOO- + H +
Initial moles | 1 | 0 | 0 |
Equilibrium moles |
(1-α) |
α |
α
|
Total moles at equilibrium = (1-α) + 2 α
= 1 + α
In order to find out the depression in freezing point,

values of I (vant Hoff's factor) and α (degree of dissoc
New answer posted
6 months agoContributor-Level 10
16.18
Dettol is a well known antiseptic. It is a mixture or combination of chloroxylenol and terpineol in a suitable solvent.
The structure of chloroxylenol is as follows:
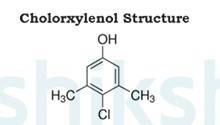
The structure of terpineol is as follows:
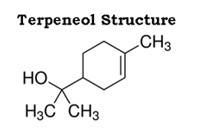
New answer posted
6 months agoContributor-Level 10
The depression in freezing point of water observed for the same amount of acetic acid, trichloroacetic acid and trifluoroacetic acid increases in the pattern,
Acetic acid< trichloroacetic acid< trifluoroacetic acid.
This is because fluorine is more electronegative than chlorine. So, trifluoracetic acid is a stronger acid in comparison to trichloroacetic acid and acetic acid. And also, acetic acid is the weakest of all.
Explanation: Stronger acid produces more number of ions, therefore it has more? Tf (depression in freezing point), hence lower freezing point. As the acidic strength increases, the acid gets more and more ionised.
Trifluoracetic acid ionizes to the largest extent. Hen
New answer posted
6 months agoContributor-Level 10
Volume of the solution = 250mL = 0.25L
Let the no. of moles of solute be n
Molarity = No. of moles of solute/volume of solution
⇒ 0.15 = n/0.25
⇒ n = 0.0375moles
Molar mass of C6H5OH = 6*12 + 5*1 + 16 + 1 = 94g
Moles = mass/molar mass
⇒ 0.0375 = m/94
Mass of benzoic acid required = 3.525g.
New answer posted
6 months agoContributor-Level 10
16.17
Phenol is one such particular substance that can act both as an antiseptic as well as a disinfectant depending upon the concentration of the solution used. An antiseptic is used to prevent the growth of germs in cuts and wounds.
For instance, a phenol of 0.2% concentration acts as antiseptic and phenol of concentration 1% acts as a disinfectant.
New answer posted
6 months agoContributor-Level 10
Molar mass of Nalorphene = 311g/mol
Now 1000g of solution contains 1.5 * 10-3 moles of Nalorphene (Molality of solution = moles of solute/mass of solvent (in kg)
⇒ 1.5 * 10-3 moles of Nalorphene = 1.5 * 10-3 * 311 = 0.4665g of Nalorphene
Therefore, total mass of the solution = (1000 + 0.4665) g
⇒ total mass = 1000.4665 g
This implies that the mass of the solution containing 0.4665 g of nalorphene is 1000.4665 g.
Therefore, mass of the solution containing 1.5 mg of nalorphene is:
Mass = 1000.4665 X 1.5 X 10-3 / 4.665 g
⇒ mass of solution containing required ions = 3.22 g
Hence, the mass of aqueous solution required is 3.22 g.
New answer posted
6 months agoContributor-Level 10
16.16
Magnesium or Aluminium hydroxides do not tackle with the root cause of acidity that is they reduce the acidity by reacting with excess acid present in the stomach [neutralization reaction].
On the other hand cimetidine and ranitidine relieve acidity by preventing interaction of histamine with receptors of stomach walls and hence release of excess acid by stomach walls is stopped. So the root cause of acidity is totally eliminated.
Therefore cimetidine and ranitidine relieve the acidity by tackling the root cause whereas magnesium or aluminum hydroxide just relieves by healing only the mere cause of acidity.
New answer posted
6 months agoContributor-Level 10
Total mass of solution = 6.5g + 450g = 456.5g
Therefore mass percentage of aspirin in solution = (mass of aspirine/total mass of solution) = 6.5/456.5
⇒ mass % of aspirine = 1.424%
New answer posted
6 months agoContributor-Level 10
16.15
Antiseptics and disinfectants are effective against micro-organisms. Antiseptics are applied to the living tissues such as wounds, cuts, ulcers, and diseased skin surfaces, while disinfectants are applied only to inanimate objects such as floors, drainage system, instruments, etc. Disinfectants are harmful to the living tissues. Iodine is an example of a strong antiseptic. Tincture of iodine (2 − 3 percent of solution of iodine in alcohol − water mixture) is applied to wounds. 1 percent solution of phenol is used as a disinfectant.
New answer posted
6 months agoContributor-Level 10
The Solubility product of CuS (ksp) = 6 * 10-16
CuS → Cu ++ + S2-
Let the s be solubility of CuS in mol/L
Ksp = [ Cu ++ ] [S2]
Ksp = solubility product
6 * 10-16 = s * s = s2
⇒ S = 2.45 * 10-8 mol/L
Hence, the maximum molarity of CuS in an aqueous solution is 2.45 * 10-8 mol/L
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers


