Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
ln
Hence; sum of oxidation state = 2
New answer posted
3 months agoContributor-Level 10
Mass of empty cylinder = 14.8 kg
Mass of cylinder when full = 29 kg
Mass of gas in cylinder when filled; W1 = 29 – 14.8 = 14.2 kg
Mass of gas in cylinder after using, W2 = 23 – 14.8 = 8.2 kg
Initial pressure ; P1 = 3.47 atm
Final pressure ; P2 =?
Using
P2 = atm
New answer posted
3 months agoContributor-Level 10
Glyptal, Dacron & PHBV are polyesters.
Novalac is copolymer of phenol & formaldehyde but not polyesters.
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
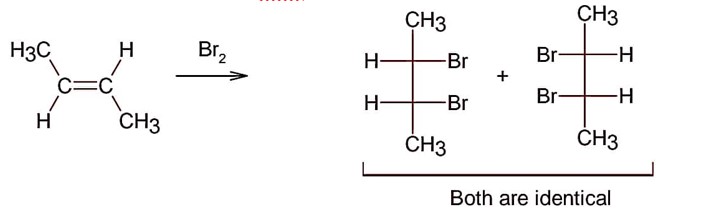
Electrophilic addition of bromine to an alkene is anti-addition, in which cis-alkene gives two enantiomers and trans – alkene gives meso form
Here; trans-but-2-ene will give meso products
New question posted
3 months agoNew question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
Na/H2 can not reduce a functional group as Na does not behaves as catalyst here.
New answer posted
3 months agoContributor-Level 10
Stability order of oxides (X2O) is,
l2O > Cl2O > Br2O
Bonds of halogen & oxygen are covalent due to less EN difference.
Stability of (I - O) bond is higher due to less polarity and that of (Cl-O) bond is higher due to multiple bonding.
New question posted
3 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers