Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
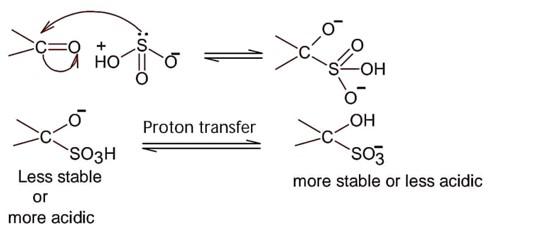
Nucleophilic addition of sodium hydrogen sulphite to aldehyde or ketone is a;
So; nucleopilic addition of sodium hydrogen sulphite to an aldehyde or a ketone involves proton transfer to form a stable ion.
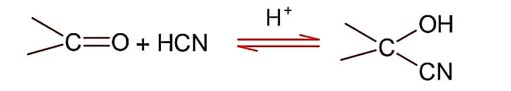
Addition of hydrogen cyanide;
Final product is cyanohydrin.
New answer posted
3 months agoContributor-Level 10
BOD is biological oxygen demand which represents the amount of oxygen required to degrade organic matter in water.
Higher the BOD more polluted the water is
So; here water sample with BOD = 3 ppm is the cleanest
New answer posted
3 months agoContributor-Level 10
Spin only moment ;
3.87 =
So, number of unpaired electrons; n = 3
ln ;
So; cannot be V3+
New answer posted
3 months agoContributor-Level 10
Impurities present in electrolytic refining of blister Cu, removed as anode mud
= Sb, Se, Te, Ag, Au, Pt
Ans. = 6
New answer posted
3 months agoContributor-Level 10
Given are the oxide of alkali and alkaline earth metals which are ionic in nature.
Simple oxide are Li2O, CaO, MgO and K2O.
Peroxide is Na2O2 and superoxide is KO2.
All simple oxides are diamagnetic as it has no unpaired electron.
New answer posted
3 months agoContributor-Level 10
Effective number of atom in C.C.P
= 4
Number of octahedral void = 4
Number of cations = 4
Number of anion = 4
Formula of compound = A4B4
Empirical formula = AB
Ans. x = 1
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers