P Block Elements
Get insights from 254 questions on P Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about P Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
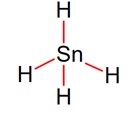
6 months agoContributor-Level 10
Stannane is the tetrahedral shape of molecule.
Hybridisation is- sp3, shape and structure are tetrahedral
New answer posted
6 months agoContributor-Level 10
P4 + 3NaOH + 3H2O ->
(It's a disproportionation reaction)
New answer posted
6 months agoContributor-Level 10
(CH3)4Si is called silane, while CH3Si (OH)3 produced 2- dimensional silicone after the removal of water, similarity
New answer posted
6 months agoContributor-Level 10
XeF2 sp3d2, square pyramidal
XeF6 sp3d3, Distorted octahedral
XeOF4 sp3d2, square pyramidal
New answer posted
6 months agoContributor-Level 10
Let a moles of SO2Cl2 is taken
Then no. of moles of H2SO4 = a moles
No. of moles of HCl = 2a moles
No. of moles of NaOH required = 2a + 2a = 4a = 16
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers