P Block Elements
Get insights from 254 questions on P Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about P Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii), (iii)
MI > MBr > MCl > MF is an iconic character of metal halide
F2 > Cl2 > Br2 > I2 is a bond dissociation enthalpy
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i) and (iii)
6NaOH+3Cl2 → 5NaCl+NaClO3+3H2O
Chlorine gas oxidation number ranges from 0 to –1 to 0 to +5.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (ii)
BrO2- (35+2*8+1=52) and BrF2+ (35+2*9−1=52)
52 electrons are present in both
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
The ability of a substance to be decreased is known as its reduction potential. As the substance's reduction potential rises, so does its power to reduce. It signifies the chemical's oxidising power (the ability of the material to cause other substances to lose electrons, which is known as oxidising power) grows.
As a result, the oxidising power is listed in decreasing order BrO4- > IO4- > ClO4-
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iv)
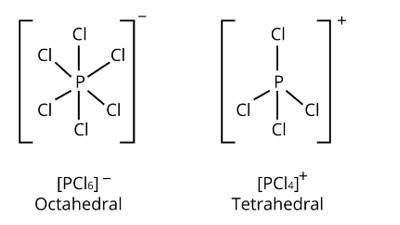
The ionic bonding promotes the crystalline structure of PCl5 in the solid state by attempting to exist as oppositely charged ions like [PCl4]+ and [PCl6]− . Also, [PCl4]+ and [PCl6]− are tetrahedral and octahedral, respectively. These structures fit together well, giving the solid structure extra stability.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
Bartlett noticed that Xenon's first ionisation potential is nearly identical to that of oxygen and used Born-Haber calculations to predict the presence of a stable compound: Xe+ Pt F6-
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iv)
MnO2 ( Black )+4HCl→MnCl2+2H2O+Cl2
Greenish yellow color
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
C+2H2SO4→CO2+2SO2+2H2O
Conc. H2SO4 oxidises C to produce two gaseous products.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (i)
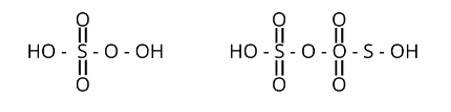
Peroxy linkage refers to the presence of a bond between oxygen and oxygen (O-O) in a molecule.
Only H2S2O6 and H2S2O7 of the sulphur oxoacids above have a peroxy linkage.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
The C-atom in the CO32- ion undergoes sp2 hybridization. BF4, NH4+ and SO42- have a tetrahedral structure, whereas it has a triangular planar structure.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers

