P Block Elements
Get insights from 254 questions on P Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about P Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
O2F2 oxidises plutonium to PuF6 and the reaction is used in removing plutonium as PuF6 from spent nuclear fuel.
New answer posted
6 months agoContributor-Level 10
When red phosphorus is heated in a sealed tube at 803 K, a - black phosphorus is formed.
New answer posted
6 months agoContributor-Level 10
(1)
(2)
(3) PbO2 + 2H2O ->Pb (OH)4
(4) SnO2 + 2H2O ->Sn (OH)4
New answer posted
6 months agoContributor-Level 10
Type of back bonding:-
Therefore order of back bonding strength =>
BF3 > BCl3 > BBr3 > Bl3
New answer posted
6 months agoContributor-Level 9
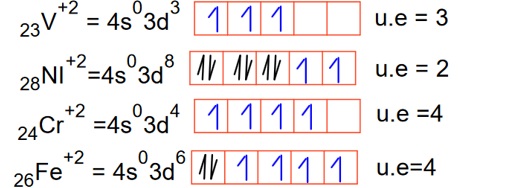
the lowest spin only magnetic moment for Ni+2 because of minimum unpaired electron.
New answer posted
6 months agoContributor-Level 10
For AB3 interhalogen compound, which is T-shaped, only two lone pair electrons are available on central atom.
New answer posted
6 months agoContributor-Level 10
Reactivity order of halogens and inter halogens are F2 > ClF > Cl2 and ClF forms HOCl and HF after the reaction with water.
New answer posted
6 months agoContributor-Level 10
Here 'X' is the Gypsum (CaSO4.2H2O) which is used to enhance the setting of time.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers