P Block Elements
Get insights from 254 questions on P Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about P Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Zone refining is used to obtain high purity elements which are used in the manufacture of semiconductors. Boron and silicon both are used in semiconductors.
New answer posted
5 months agoContributor-Level 9
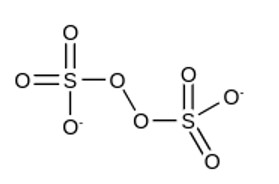
Number of bond between sulphur and oxygen
Number of bond between sulphur and sulphur
New answer posted
6 months agoContributor-Level 10
Compound Oxidation state of P
H? P? O? → 5
H? P? O? → 3
H? P? O? → 4
New answer posted
6 months agoContributor-Level 10
Hydrogen peroxide reduces iodine to iodide ion is basic medium as;
New answer posted
6 months agoContributor-Level 10
Beo & Be (OH)2 are amphoteric in nature, because they react with both acid and base
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers