P Block Elements
Get insights from 254 questions on P Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about P Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
Both (A) and (B) are correct but R is not correct explanation of A. In both
New answer posted
6 months agoContributor-Level 10
It does not contains 2nd most abundant element by weight in earth crust because that is Si Calgon ->Na2 [Na4 (PO3)6]
New answer posted
6 months agoContributor-Level 10
Blue cupric metaborate is reduced to cuprous metaborate in a luminous flame.
Cupric metaborate is obtained by heating boric anhydride & copper sulphate in a non-luminous flame as
New answer posted
6 months agoContributor-Level 10
α - sulphur & β - sulphur – Diamagnetic, S2 – form is paramagnetic due to presence of unpaired electron in π* orbital like O2.
New answer posted
6 months agoContributor-Level 10
Blood – Negatively charged colloid.
According to Hardy – Schulze Rule
FeCl3 is more efficient for blood clotting
New answer posted
6 months agoContributor-Level 10
(-1) oxidation (0)
Here, I- is reducing agent
New answer posted
6 months agoContributor-Level 10
All given oxide have nitrogen – nitrogen bond except N2O5 as ;
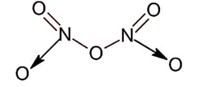
New answer posted
6 months agoContributor-Level 10
Let a moles of SO2Cl2 is taken
Then no. of moles of H2SO4 = a moles
No. of moles of HCl = 2a moles
No. of moles of NaOH required = 2a + 2a = 4a = 16
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
