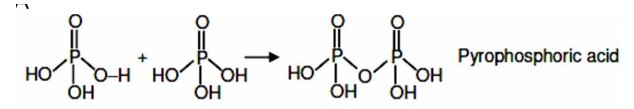
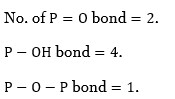P Block Elements
Get insights from 254 questions on P Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about P Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 9
Aqua regia is a mixture of concentrated nitric acid (HNO?) and concentrated hydrochloric acid (HCl), optimally in a molar ratio of 1:3. The reaction between them produces nitrosyl chloride (NOCl), chlorine gas (Cl?), and water. The potent oxidizing nature comes from these products, which can dissolve noble metals like gold and platinum. The overall reaction often shows the formation of nitric oxide (NO).
HNO? + 3HCl → NOCl + Cl? + 2H?O
The nitrosyl chloride can further decompose: 2NOCl → 2NO + Cl?. The gas evolved in the process is Nitric Oxide (NO).
New answer posted
5 months agoContributor-Level 10
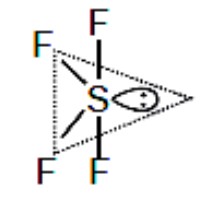
. [XeF? ]? has 7 electron pairs (5 bonding, 2 lone), pentagonal planar. XeO? F? has 5 electron pairs (trigonal bipyramidal).
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers