Amines
Get insights from 72 questions on Amines, answered by students, alumni, and experts. You may also ask and answer any question you like about Amines
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
a month agoContributor-Level 6
Amines are bases because they have a lone pair of electrons on nitrogen and their nucleophilic nature (they are electron-rich, so they are attracted to electron-deficient species to donate electrons and accept protons.
New answer posted
a month agoContributor-Level 6
In Amines, the nitrogen atom bonds with alkyl or aryl groups replacing hydrogen, whereas in amides, the nitrogen atom bonds directly with the carbonyl group (-CO-).
New answer posted
a month agoContributor-Level 10
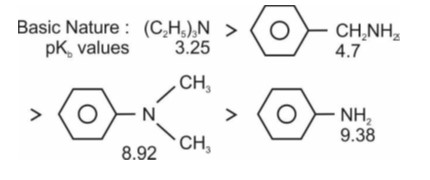
Correct order of basic strength in aqueous medium is
(CH3)2NH > CH3NH2 > (CH3)3N > NH3
New answer posted
3 months agoContributor-Level 9
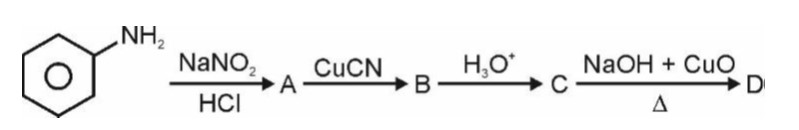
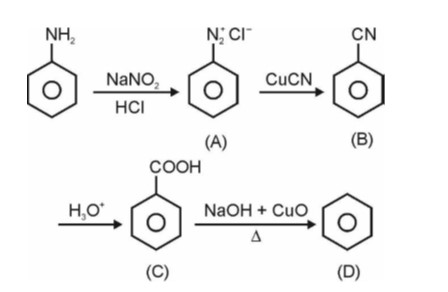
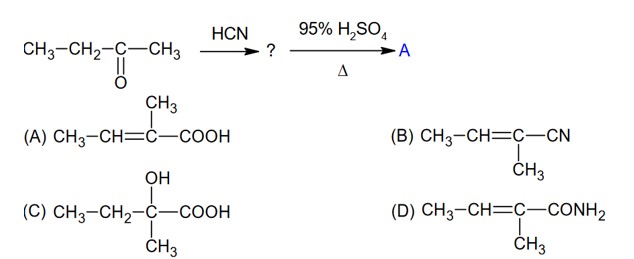
The chapter, Chapter 9, Amines, contains a 6 marks (it has a regular weightage) in the theory paper consisting of 70 marks in CBSE Class 12 Chemistry. This qualifies it to be a significant chapter under the Organic Chemistry section which as a whole obtains the most weightage. Amines discusses topics of nomenclature, classification, structure, preparation, physical and chemical properties, uses and also identification of primary, secondary and tertiary amines and the Diazonium salts.
New answer posted
3 months agoContributor-Level 10
HOCl produce in the stratospheric cloud, by the hydrolysis reaction of ClONO2.
New answer posted
3 months agoContributor-Level 10
Ceric ammonium nitrate is used to test alcohol while CHCl3/alc. KOH is used to test 1° amine
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers