Amines
Get insights from 162 questions on Amines, answered by students, alumni, and experts. You may also ask and answer any question you like about Amines
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
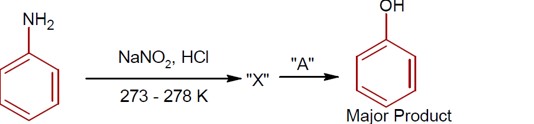
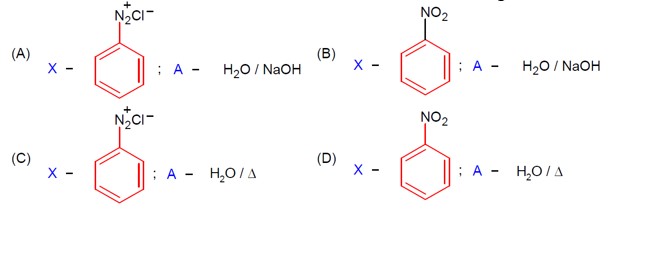
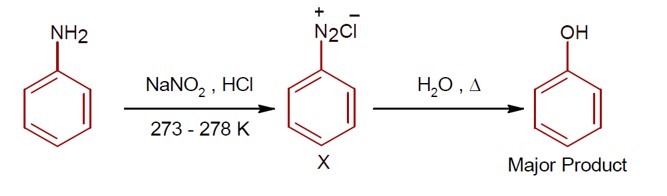
Aliphatic diazonium salts are not stable. Aromatic diazonium salts exist at low temp of 0 – 4°C.
New answer posted
4 months agoContributor-Level 9
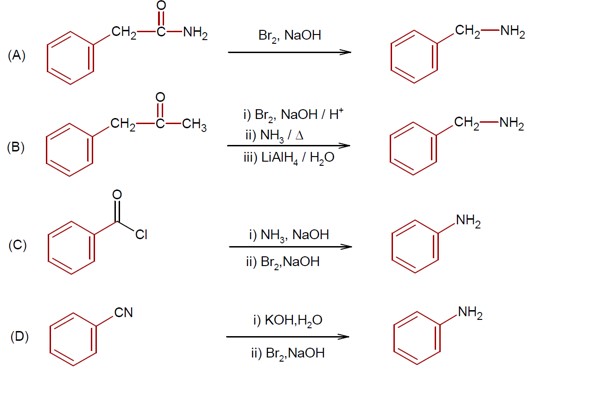
R-CONH? + Br? + 4NaOH → R-NH? + 2NaBr + Na? CO? + 2H? O
This reaction is the Hoffmann bromamide degradation, in which an amide is converted to a 1° amine.
New answer posted
4 months agoContributor-Level 10
In diacetamide (CH? CO)? NH), the lone pair of electrons on the nitrogen atom is delocalized through resonance with both adjacent carbonyl groups. This extensive resonance greatly decreases the electron density on the nitrogen atom.
New answer posted
4 months agoContributor-Level 9
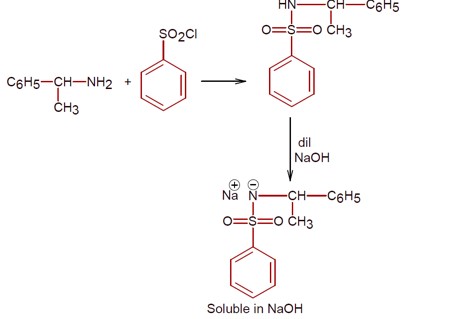
Only 1° amine react with benzene sulphonyl chloride to give a compound which is soluble in alkali
New answer posted
4 months agoContributor-Level 10
In the ammonolysis reaction, HCl is produced as a byproduct. To neutralize this acidic impurity, the mixture is treated with NaOH.
New answer posted
4 months agoContributor-Level 9
In Hoffmann bromamide reaction, hypobromite ion react with amide and in this reaction carbonyl group is lost as CO? ²? in form of Na? CO?
New answer posted
5 months agoContributor-Level 10
In the ammonolysis process, bond cleavage is carried out in the presence of NH? When a halide compound is treated with NH? , the halide ion (X? ) is substituted by an amino group (NH? ) in a nucleophilic substitution reaction.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 685k Reviews
- 1800k Answers