Amines
Get insights from 162 questions on Amines, answered by students, alumni, and experts. You may also ask and answer any question you like about Amines
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
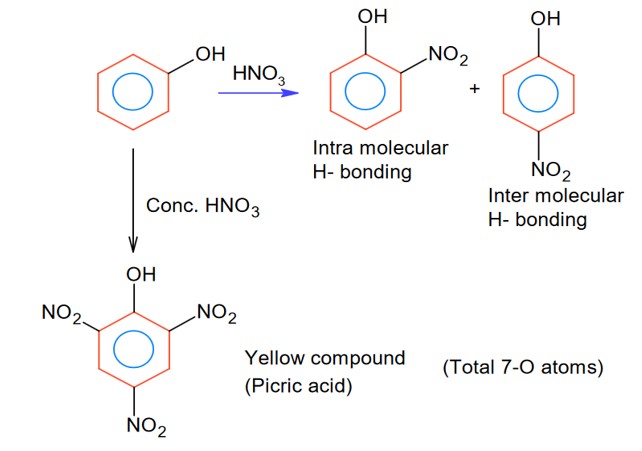
Intermolecular H- bonding and intra-molecular H- bonding producing compound may be the phenol derivatives.
New question posted
6 months agoNew question posted
6 months agoNew answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
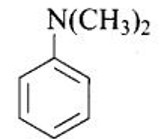
Ans: N, N-dimethylbenzenanmine
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans:
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: CH3CH2CH3 < CH3CH2NH2 < CH3CH2OH
As oxygen is more electronegative than nitrogen therefore, the O-H bond is more polar than the N-H bond. So ethanol has more dipole moments than ethylamine. Propane is non- polar in nature hence, had the least among all.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: CS2 is a non-polar solvent which decreases the activating effect of? NH2. As a result, mono substitution occurs only at o- and p- positions giving a mixture of 2-bromoaniline (minor) and 4-bromoaniline (major) as products.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: The azo products have an extended conjugate system with both aromatic rings linked by the –N=N- bond. These compounds are frequently colored and used as dyes. Benzene diazonium chloride reacts with phenol to form p-hydroxy azobenzene by coupling the phenol molecule in its para position with the diazonium salt. This is referred to as a coupling reaction.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: Amides are the byproducts of the acylation reaction. The reaction is carried out in the presence of a stronger base than the amine, such as pyridine, which removes the formed HCl and shifts the equilibrium to the right.
New answer posted
7 months agoContributor-Level 10
This is a Short Type Questions as classified in NCERT Exemplar
Ans: As the electronegativity of oxygen is more than the electronegativity of a nitrogen atom, the O−H bond is more polar than the N−H bond, therefore MeOH is stronger acid than MeNH2 or MeNH2 is stronger base than MeOH.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 685k Reviews
- 1800k Answers