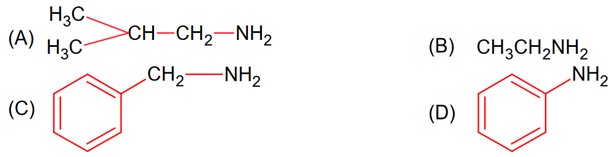Amines
Get insights from 162 questions on Amines, answered by students, alumni, and experts. You may also ask and answer any question you like about Amines
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Gabriel phthalimide synthesis is used for 1° Aliphatic /alicyclic amine
1° Amine
CH3 – CH2 – NH2
New answer posted
5 months agoContributor-Level 10
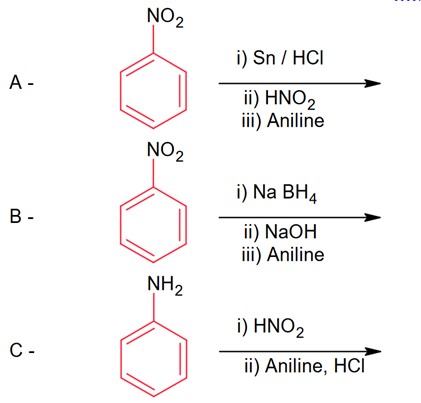
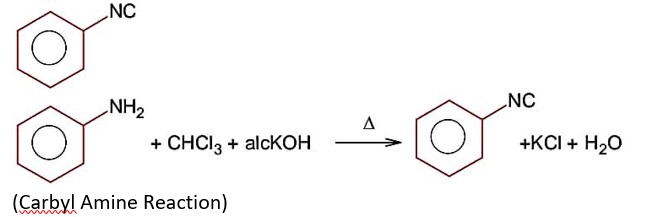
Primary amine, whether aliphatic or aromatic when warmed with CHCl3, alcoholic (KOH) it forms isocyanide
New answer posted
5 months agoContributor-Level 10
Mass of empty cylinder = 14.8 kg
Mass of cylinder when full = 29 kg
Mass of gas in cylinder when filled; W1 = 29 – 14.8 = 14.2 kg
Mass of gas in cylinder after using, W2 = 23 – 14.8 = 8.2 kg
Initial pressure ; P1 = 3.47 atm
Final pressure ; P2 =?
Using
P2 = atm
New answer posted
5 months agoContributor-Level 10
Dumas method,
Moles of N in N, N-dimethylaminopentane (C7H17N)
= 11.25 mol
= 1125 * 10-2 mol
Ans. = 1125
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 685k Reviews
- 1800k Answers