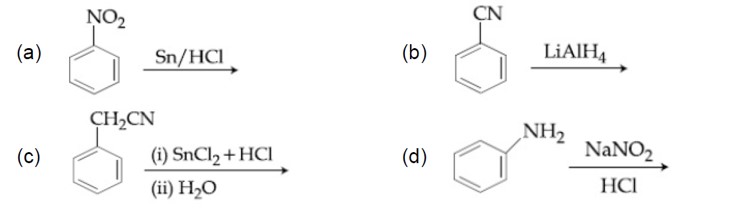
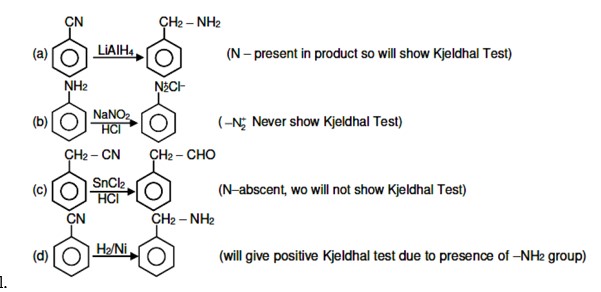
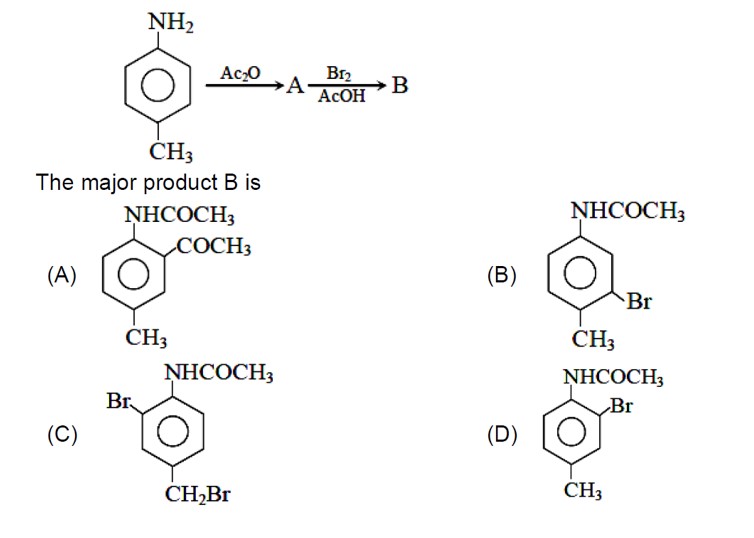
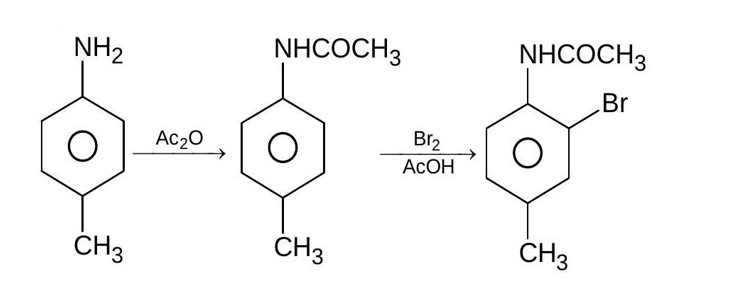
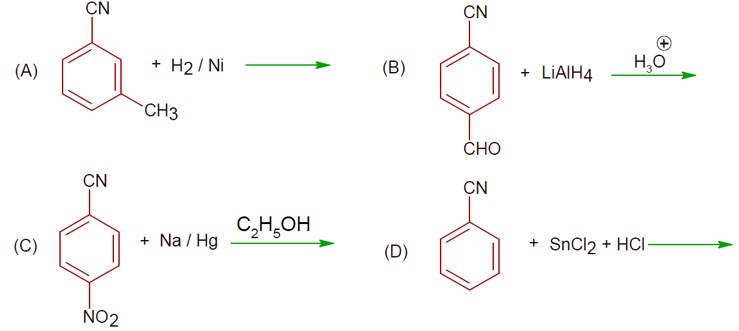
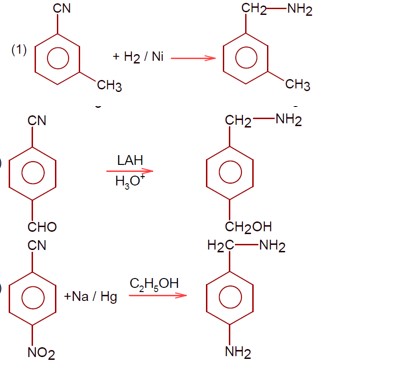
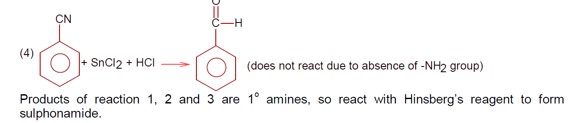
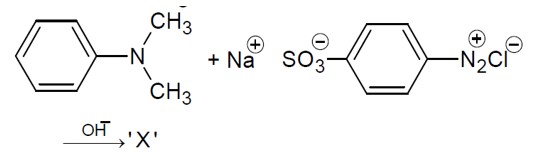Amines
Get insights from 162 questions on Amines, answered by students, alumni, and experts. You may also ask and answer any question you like about Amines
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Due to inter molecular -Bonding in , than is more soluble and having more B.P point than A.
New answer posted
5 months agoContributor-Level 10
In triethyl amine, nitrogen is sp³ hybridized, hence bond angle is approximately 109°28' but since lone pair- bond pair repulsion is greater than bp-bp repulsion. Hence, the exact angle is found to be 108°.
New answer posted
5 months agoContributor-Level 10
In Aniline, lone pair is delocalized on less EN carbon atom while in acetamide it is delocalized on more EN oxygen atom. Hence aniline is more basic than acetamide.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 685k Reviews
- 1800k Answers