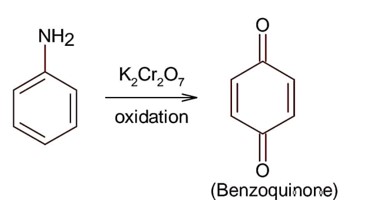
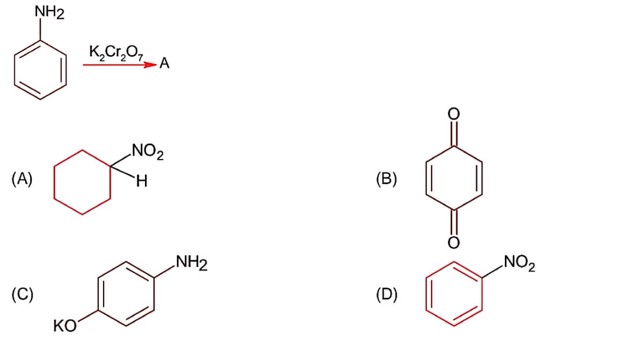
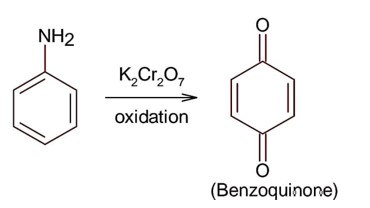
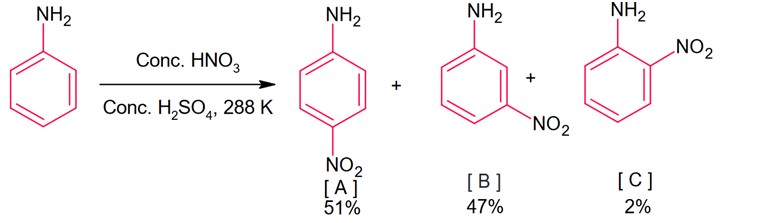
Amines
Get insights from 162 questions on Amines, answered by students, alumni, and experts. You may also ask and answer any question you like about Amines
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
% of N = (1.4 * Normality of acid * volume of acid used) / (mass of organic compound)
42 = (1.4 * (1 * 2) * volume of acid used) / 0.8
Volume of acid used = (42 * 0.8) / (1.4 * 2) ml
New answer posted
5 months agoContributor-Level 9
(CH? )? N? HCl produces H? which reacts with NaHCO? and CO? gas evolved, because HCl is stronger acid than H? CO?
New answer posted
5 months agoContributor-Level 9
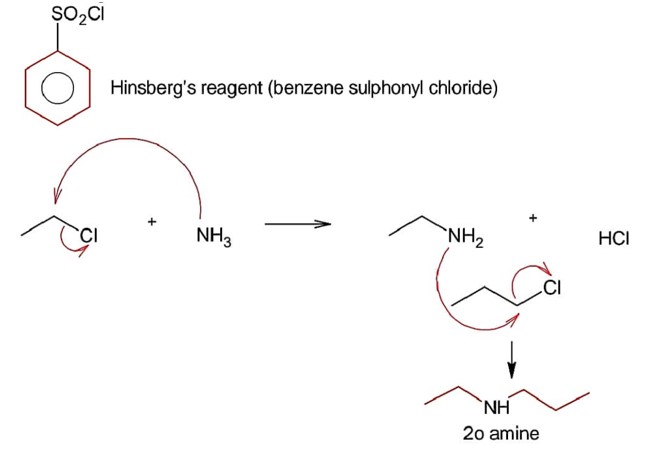
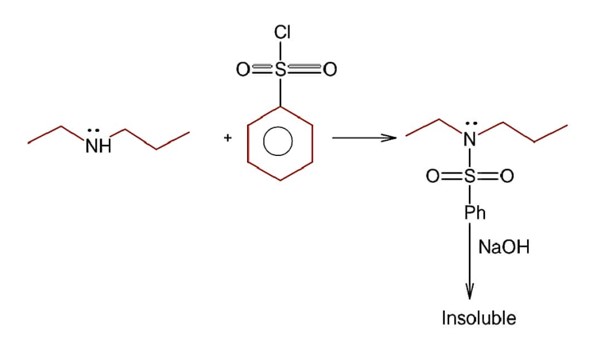
Gabriel phthalimide is used for the preparation of 1° aliphatic amine not 1° aromatic amines since 1° aromatic amines do not undergo nucleophilic substitution reaction.
New answer posted
5 months agoContributor-Level 10
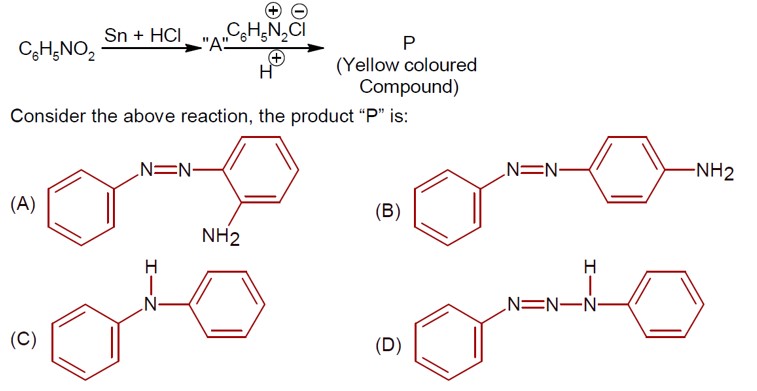
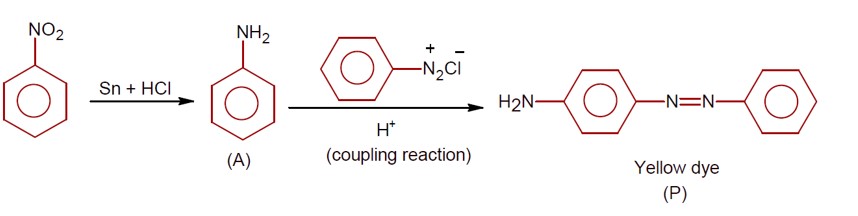
Only 1° aromatic amines give stable diazonium salt on reaction with nitrous acid
Here 1° aromatic amine is 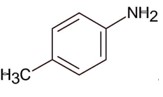
New answer posted
5 months agoContributor-Level 10
During nitration of aniline, meta product is also formed, it is because of due to presence of anilinium ion. In anilium group is meta directing group.
New answer posted
5 months agoContributor-Level 10
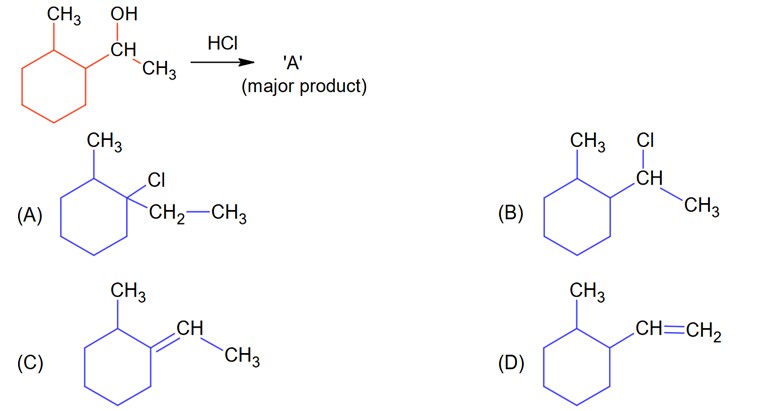
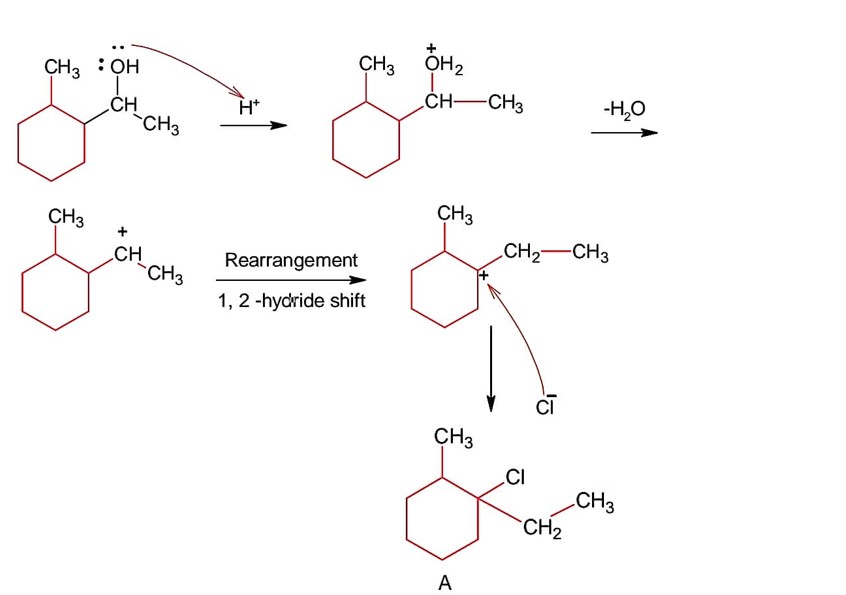
A > B > C > D
Lone pair is localized in (A) while all 3 have delocalized lone pair but they can be compared by 3° > 2° > 1° because methyl group increases the basicity.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 685k Reviews
- 1800k Answers